
Preparation and photocatalytic activity of nano-TiO2 codoped with fluorine and ferric
LI Fa-tang(李发堂)1, LIU Rui-hong(刘瑞红)1, ZHAO Di-shun(赵地顺)2,
SUN Zhi-min(孙智敏)1, QU Zhi-ming(曲志明)3
1. College of Science, Hebei University of Science and Technology, Shijiazhuang 050018, China;
2. College of Chemical and Pharmaceutical Engineering,Hebei University of Science and Technology, Shijiazhuang 050018, China;
3. School of Civil Engineering, Hebei Engineering University, Handan 056038, China
Received 15 July 2007; accepted 10 September 2007
Abstract: Nano-F-/Fe3+/TiO2 particles were prepared by hydrolysis of tetrabutyl titanate in a mixed CF3COOH-Fe(NO3)3-H2O solution. The photocatalytic decomposition of methylene blue in aqueous solution was used as a probe to evaluate their photocatalytic activities. The powders were characterized by X-ray diffraction (XRD), energy dispersion X-ray spectrum (EDS) and Brunauer-Emmett-Teller (BET) surface area analysis. The results show that F- and Fe 3+ are doped into TiO2. The F- and Fe3+ doping can help to enhance the nano-TiO2 photocatalytic activity greatly. The appropriate codoping conditions for F-Fe are n(F)/n(TiO2)=2%, n(Fe)/n(TiO2)=0.05%, and the degradation rate of methylene blue at 1 h is improved from 73.2% to 87.5%. The codoped nano-F-/Fe3+/TiO2 particles have higher BET specific surface area, smaller crystallite size and higher photocatalytic activity than those of undoped TiO2 particles.
Key words: nano-TiO2; F-/Fe3+/TiO2; codoping; photocatalytic activity
1 Introduction
Titanium dioxide is widely used as a photocatalyst because of its photochemical stability, non-toxicity and low-cost[1]. However, the low efficiency for the utilization of visible light and recombination between the photogenerated electrons and holes are often two major limiting factors that impede the enhancement of photocatalytic activity. Many studies have been devoted to the improvement of photocatalytic efficiency of TiO2, such as doping metal or non-metal ions[2-4], depositing noble metals[5-6], surface sensitization[7] and composite semiconductor[8-9].
Doping transition metals or non-metal ions are effective method to improve photocatalytic activity of nano-TiO2. XIN et al[10] revealed the mechanisms of photoinduced carriers separation and recombination of Fe3+-TiO2 photocatalysts, that is, Fe3+ captured the photoinduced electrons, inhibiting the recombination of photoinduced electron-hole pairs. YU et al[11] prepared F--doped TiO2 powders by hydrolysis of titanium tetraisopropoxide in a mixed NH4F-H2O solution and found that photocatlytic activity of F--doped TiO2 powders exceeded that of Degussa P25 when the molar ratio of NH4F to H2O was kept in the range of 0.5-3.0.
In this work, F- and Fe3+-codoped TiO2 nanoparticles were prepared from tetrabutyl titanate through sol-gel method, characterized by means of X-ray diffraction (XRD), energy dispersion X-ray spectrum (EDS) and Brunauer-Emmett-Teller (BET) surface area analysis. The photocatalytic activities of the prepared powders were studied following the degradation of methylene blue.
2 Experimental
2.1 Preparation of F--Fe3+-TiO2 nanoparticles
Specimens of F--Fe3+-TiO2 containing different amounts of F- and Fe3+ were prepared by sol–gel method.10 mL of Ti(OC4H9)4 was added slowly into mixed solution containing 15 mL C2H5OH and 4.5 mL CH3COOH to form solution A. Meanwhile CF3COOH and Fe(NO3)3?9H2O were added into mixed solution containing 4 mL water, 7.5 mL C2H5OH and 4.5 mL CH3COOH to form solution B. Then the solution B was added dropwise into the solution A under vigorous stirring at room temperature. The resulting transparent colloidal liquid was continuously stirred till the gel was formed. The gel was dried at 110 ℃ for 6 h, calcined at 450 ℃ for 2 h in air and ground to obtain the F--Fe3+-TiO2 nanoparticles. The molar ratios of F to Ti are 0 and 2%, and the molar ratios of Fe3+ to Ti are 0, 0.01%, 0.03%, 0.05%, 0.1% and 0.15%.
2.2 Characterization of samples
The XRD patterns obtained on a BRUKER D8-ADVANCE X-ray diffractometer using Cu Kα radiation (λ=0.154 2 nm) at a scan rate of 0.05?/s were used to determine the crystal structure and crystallite size. The accelerating voltage and the applied current were 15 kV and 20 mA, respectively. The crystallite size of the anatase was determined by applying Scherrer equation to XRD data. Energy dispersive X-ray spectroscopy (EDS) was recorded on a PHILIP XL-30 ESEM. The BET surface area was determined using a specific surface area analysis meter (Quantachrome NOVA2000).
2.3 Photocatalytic activity measurement
The photocatalytic degradation of methylene blue over photocatalysts was carried out in XPA-Ⅱ photochemical reactor (Nanjing Xujiang Electromechanical Factory). A 500 W high-pressure mercury lamp was used as light source, whose photo intensity was 0.22 kW?m-2 at 365 nm. In each run, 0.05 g photocatalysts were added into 100 mL methylene blue solution of 13 mg?L-1. After premixing for 5 min, the light was turned on to initiate the reaction for 1 h. A 723 spectrometer was used to determine the concentration of methylene blue solution before and after photocatalytic degradation.
3 Results and discussion
3.1 EDS spectrum
The EDS spectrum of the F--Fe3+-TiO2 sample with molar ratios of F and Fe to Ti of 2% and 0.05% is shown in Fig.1. The EDS spectrum indicates that there are F and Fe elements in the sample, that is, F- and Fe3+ can be doped into TiO2 through hydrolysis of tetrabutyl titanate by using CF3COOH and Fe(NO3)3 as dopant.
3.2 XRD and BET analysis
Fig.2 shows the XRD patterns of TiO2 and F--Fe3+-TiO2 sample, in which molar ratios of F and Fe to Ti are 2% and 0.05%. It can be seen that the diffraction peaks of samples are all ascribed to the anatase phase.
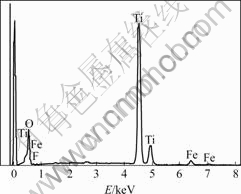
Fig.1 EDS spectrum of F-/Fe3+/TiO2
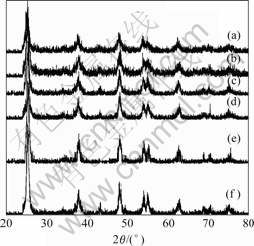
Fig.2 XRD patterns of TiO2 and F--Fe3+-TiO2 powders calcined at 450 ℃ for 2 h: (a) 2%F-0.15%Fe-TiO2; (b) 2%F-0.10%Fe- TiO2; (c) 2%F-0.05%Fe-TiO2; (d) 2%F-0.03%Fe-TiO2; (e) 2%F-0.01%Fe-TiO2; (f) TiO2
With the increase of Fe-doping concentration, the peak intensities of anatase slightly decrease and the width of plane diffraction peak becomes broader. Therefore, it is reasonable to deduce that the larger the amount of Fe-doping, the poorer the crystallization of the TiO2 powders and the smaller the crystallite size of TiO2. The results are the same as those of the BET surface area analysis shown in Table 1. It can be seen that the codoping of F- and Fe3+ can inhibit the growth of TiO2 crystal.
3.3 Evaluation of photocatalytic activity
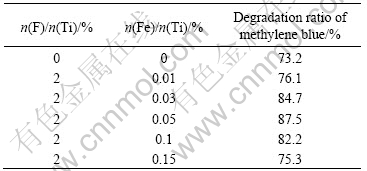
Table 2 lists the photocatalytic degradation rate of methylene blue over F--Fe3+-TiO2 photocatalysts with n(F)/n(Ti) of 2% and different Fe3+ dopant contents. F--doping can convert some Ti4+ to Ti3+ by charge compensation[11-12]:
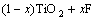 →
→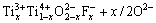
Table 1 Effects of Fe-doping concentration on physical properties of TiO2 powders with n(F)/n(TiO2) of 2%
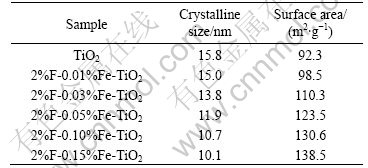
Table 2 Degradation ratio of methylene blue by codoped TiO2 with different concentrations of F- and Fe3+
Since TiO2 is an n-type semiconductor, Ti3+ in TiO2 forms a donor level between the band gaps of TiO2. Ti3+ may trap the photogenerated electrons and then reduce recombination rate of electrons and holes and enhances photocatalytic activity. In the work, the molar ratio of F to Ti is fixed at 2%.
It can also be found that the photodegradation ratio of methylene blue increases with the increase of content of Fe3+ dopant. When the content of Fe3+-TiO2 is over 0.05%(molar fraction), the degradation ratio of methylene blue is the highest, which can be attributed to the following reasons. Appropriate amount of the doped Fe3+ (≤0.05%) in TiO2 can effectively capture the photoinduced electrons, which inhibits the holes-electrons recombination and induces more photo-generated electrons and holes to participate in the photocatalytic reactions. When the content of Fe3+ dopant exceeds 0.05%(atom fraction), it can be seen that the degradation ratio decrases. The reason is that the photocatalytic activity of Fe-doped TiO2 is strongly dependent on the dopant concentration since Fe3+ can serve as not only a mediator of interfacial charge transfer but also a recombination center[10, 13]. In this case, an optimal dopant concentration is 0.05%(molar fraction). Above that concentration, Fe3+ steadily becomes recombination center of the photoinduced electrons and holes, which is unfavorable to photocatalytic reactions.
4 Conclusions
1) Nano-F-/Fe3+/TiO2 particles are prepared by hydrolysis of tetrabutyl titanate in a mixed CF3COOH- Fe(NO3)3-H2O solution. The EDS spectrum indicates that there are F and Fe elements in the sample.
2) At an optimal concentration of n(Fe)/n(Ti)= 0.05% and n(F)/n(Ti)=2% , the photocatalytic activity of F--Fe3+-TiO2 powders prepared by this method and calcined at 450 ℃ shows the highest activity. The degradation rate of methylene blue at 1 h is improved from 73.2% to 87.5%.
3) The codoping of F- and Fe3+ can inhibit the growth of TiO2 crystal. Nano-F-/Fe3+/TiO2 particles have higher BET specific surface area and smaller crystallite size than those of undoped TiO2 particles.
References
[1] NAGAVENI K, SIVALINGAM G, HEGDE M S, MADRAS G. Photocatalytic degradation of organic compounds over combustion-synthesized nano-TiO2[J]. Environ Sci Technol, 2004, 38(5): 1600-1604.
[2] WEN Chen, DENG Hua, TIAN Jun-ying ZHANG Ji-mei. Photocatalytic activity enhancing for TiO2 photocatalyst by doping with La[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(S2): 728-731. (in Chinese)
[3] REYES-GARCIA E A, SUN Y, REYES-GIL K. 15N solid state NMR and EPR characterization of N-Doped TiO2 photocatalysts[J]. Journal of Physical Chemistry C, 2007, 111(6): 2738-2748.
[4] PINZARI F, PATRONO P, COSTANTINO U. Methanol reforming reactions over Zn/TiO2 catalysts[J]. Catalysis Communications, 2006, 7(9): 696-700.
[5] BOWKER M, JAMES D, STONE P, BENNETT R, PERKINS N, MILLARD L, GREAVES J, DICKINSON A. Catalysis at the metal-support interface exemplified by the photocatalytic reforming of methanol on Pd/TiO2[J]. J Catal, 2003, 217(2): 427-433.
[6] EINAGA H, IBUSUKI T, FUTAMURA S. Improvement of catalyst durability by deposition of Rh on TiO2 in photooxidation of aromatic compounds[J]. Environ Sci Technol, 2004, 38(1): 285-289.
[7] LUO X S, ZHA C J, LUTHER-DAVIES B. Photosensitivity of titania-doped hybrid polymer prepared by an anhydrous sol-gel process[J]. Optical Materials, 2005, 27(8): 1461-1466.
[8] DIWALD O, THOMPSON T L, ZUBKOV T, GORALSKI E G, WALCK S D, YATES J T. Photochemical activity of nitrogen-doped rutile TiO2(110) in visible light[J]. J Phys Chem B, 2004, 108(19): 6004-6008.
[9] BRYAN J D, HEALD S M, CHAMBERS S A, GAMELIN D R. Strong room-temperature ferromagnetism in Co2+-doped TiO2 made from colloidal nanocrystals[J]. J Am Chem Soc, 2004, 126(37): 11640-11647.
[10] XIN Bai-fu, REN Zhi-yu, WANG Peng, LIU Jia , JING Li-qiang, FU Hong-gang. Study on the mechanisms of photo induced carriers separation and recombination for Fe3+-TiO2 photocatalysts[J]. Applied Surface Science, 2007, 253(9): 4390-4395.
[11] YU J C, YU Jia-guo, HO W, JIANG Zi-tao, ZHANG Li-zhi. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders[J]. Chem Mater, 2002, 14(9): 3808-3816.
[12] LIN H, KUMON S, KOZUKA H, YOKO T. Electrical properties of sol-gel-derived transparent titania films doped with ruthenium and tantalum[J]. Thin Solid Films, 1998, 315(1/2): 266-272.
[13] ZHOU Ming-hua, YU Jia-guo, CHENG Bei. Effects of Fe-doping on the photocatalytic activity of mesoporous TiO2 powders prepared by an ultrasonic method[J]. Journal of Hazardous Materials B, 2006, 137(3): 1838-1847.
(Edited by CHEN Can-hua)
Foundation item: Project(203364) supported by the Natural Science Foundation of Hebei Province, China; project(XL2006038) supported by the Natural Science Foundation of Hebei University of Science and Technology, China
Corresponding author: LI Fa-tang; Tel: +86-311-88632235; E-mail: lifatang@126.com