盐酸浸出-硫酸化焙烧和水浸出从镍钼矿中提取钼和镍
来源期刊:中国有色金属学报(英文版)2017年第1期
论文作者:羡鹏飞 周升帆 王明玉 王学文 陈边防
文章页码:220 - 226
关键词:钼;镍;镍钼矿;盐酸浸出;硫酸化焙烧;水浸出
Key words:molybdenum; nickel; Ni-Mo ore; hydrochloric acid leaching; sulphation roasting; water leaching
摘 要:为了从焙烧的镍钼矿中提取钼和镍,研究了盐酸浸出-硫酸化焙烧和水浸出处理焙烧后镍钼矿的过程。实验结果表明,焙烧的镍钼矿经过盐酸浸出-硫酸化焙烧然后水浸出后能够获得高的钼和镍浸出率。氧化焙烧的镍钼矿添加0.219 mL/g盐酸(12 mol/L)在液固比为3 mL/g的条件下于65 °C浸出30 min;浸出渣添加51.9%浓硫酸在240 °C下焙烧1 h;焙砂物料采用已经获得的第一段盐酸浸出液在95 °C浸出2 h,钼和镍的总浸出率分别达到95.8%和91.3%。
Abstract: To extract molybdenum and nickel from the roasted Ni-Mo ore, a process of hydrochloric acid leaching, sulphation roasting and water leaching was investigated. The results showed that this process could get a high leaching rate of Mo and Ni. Under the optimum conditions of hydrochloric acid leaching (roasted Ni-Mo ore leached with 0.219 mL/g hydrochloric acid addition at 65 °C for 30 min with a L/S ratio of 3 mL/g), sulphation roasting (51.9% sulfuric acid addition, roasting temperature 240 °C for 1 h), followed by leaching with the first stage hydrochloric acid leaching solution at 95 °C for 2 h, the leaching rates of Mo and Ni reached 95.8% and 91.3%, respectively.
Trans. Nonferrous Met. Soc. China 27(2017) 220-226
Peng-fei XIAN, Sheng-fan ZHOU, Ming-yu WANG, Xue-wen WANG, Bian-fang CHEN
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 17 November 2015; accepted 25 August 2016
Abstract: To extract molybdenum and nickel from the roasted Ni-Mo ore, a process of hydrochloric acid leaching, sulphation roasting and water leaching was investigated. The results showed that this process could get a high leaching rate of Mo and Ni. Under the optimum conditions of hydrochloric acid leaching (roasted Ni-Mo ore leached with 0.219 mL/g hydrochloric acid addition at 65 °C for 30 min with a L/S ratio of 3 mL/g), sulphation roasting (51.9% sulfuric acid addition, roasting temperature 240 °C for 1 h), followed by leaching with the first stage hydrochloric acid leaching solution at 95 °C for 2 h, the leaching rates of Mo and Ni reached 95.8% and 91.3%, respectively.
Key words: molybdenum; nickel; Ni-Mo ore; hydrochloric acid leaching; sulphation roasting; water leaching
1 Introduction
Molybdenum and nickel have strategic and industrial importance due to their applications in ferrous metallurgy, nonferrous metal metallurgy, chemical industry and other technological fields [1]. In China, Ni-Mo ore is an important molybdenum and nickel source, and the contents of nickel and molybdenum vary in the range of 0.17%-7.03% and 0.35%-8.17%, respectively [2,3].
Several mineral processing treatments have been tested on Ni-Mo ore, such as flotation separation or gravity separation combined with flotation [4]. However, due to low metals extraction, the application of mineral dressing does not suit the separation and extraction of molybdenum and nickel from the ore.
The traditional process for molybdenum and nickel extraction from Ni-Mo ore is oxidizing roasting and melting reduction, and the product was high impurity Ni-Mo-Fe alloy [5]. This technology has two main problems, low recovery of molybdenum (<80%) and production of low value Ni-Mo-Fe alloy. In recent years, several other processes have been proposed to recover molybdenum from Ni-Mo ore, such as direct leaching with NaOH and NaClO [6], oxygen pressure NaOH leaching [7,8], roasting followed by NaOH and Na2CO3 leaching [9], leaching molybdenum by air oxidation in alkali solution [10,11] and sodium hypochlorite leaching under mechanical activation [12]. The molybdenum leaching rate in these methods is high in all cases, but nickel cannot be extracted.
To extract both of molybdenum and nickel from Ni-Mo ore, pressure acid leaching technology and HCl leaching of Ni-Mo ore with sodium chlorate were developed [13,14]. The Ni leaching rates of the two technologies are both more than 92%; however, the leaching rate of molybdenum is below 80%. For further recovery molybdenum, a alkali leaching process must be followed after acid leaching.
In Ni-Mo ore, the molybdenum mainly occurs in the form of MoS2 and nickel mainly occurs as NiS2, Ni3S4 and NiS [15]. And therefore, no matter acid leaching or alkali leaching, an oxidation process was needed. Oxidizing roasting is one of the commonly used ways of oxidation. After roasting, powellite and trevorite were generated. They can be decomposed by sulphation roasting; however, other phases hindered the contact of sulfuric acid with them, which caused the molybdenum and nickel leaching rates not be to further improved [16]. And therefore, hydrochloric acid leaching, sulphation roasting and water leaching were proposed. In the first step, hydrochloric acid leaching makes some impurities and molybdenum go into the solution, and then sulphation roasting and water leaching promote the leaching of nickel and molybdenum. Because the process of sulphation roasting has been studied in detail [17], the hydrochloric acid leaching process as well as the effect of hydrochloric acid leaching and sulphation roasting on total leaching of Mo and Ni was the focus in this work.
2 Experimental
2.1 Materials
The roasted Ni-Mo ore used in this work was taken from Fengyun Mining Co., Ltd., Hunan province of China, which was obtained under the condition of oxidation roasting Ni-Mo ore at 650-700 °C for 4 h in rotary kiln. After being ground, the particle size of roasted Ni-Mo ore is less than 0.089 mm accounting for 80%. X-ray fluorescene (XRF) was used for chemical analysis of the sample. The results showed that the roasted Ni-Mo ore mainly consisted of Si, Fe, Ca, S and O (see Table 1). All the reagents used in leaching, sulphation roasting and chemical analysis, including hydrochloric acid (12 mol/L), sulfuric acid, sodium hydroxide, etc, were of analytical grade.
The content of Mo in both roasted Ni-Mo ore and leaching residue was measured by a thiocyanate colorimetric method [18]. The analyses of nickel were carried out by atomic absorption spectrometry. X-ray diffraction (XRD) patterns were recorded by a Rigaku D/max2550VB+18 kW diffractometer with Cu Kα X-ray radiation at 40 kV and 50 mA. Figure 1 shows the XRD pattern of the roasted Ni-Mo ore.
It can be seen from Fig. 1 that the mineral compositions of the roasted Ni-Mo ore are quartz (SiO2), trevorite (NiFe2O4), hematite (Fe2O3), anhydrite (CaSO4) and powellite (CaMoO4).
2.2 Procedure
80 g roasted Ni-Mo ore and a predetermined volume of hydrochloric acid solution were charged for leaching in a 500 mL agitated flask heated by an electric hot jacket. In hydrochloric acid leaching process, the liquid to solid (L/S) ratio refers to the ratio of the total volume of water and 12 mol/L hydrochloric acid to solid quality. After the required time, the mixture consisting of leaching solution and leaching residue was filtered. The leaching residue cake was further used to extract nickel and molybdenum by sulphation roasting.
In sulphation roasting process, the leaching residue cake was mixed with a desired 98% sulfuric acid in an alumina crucible, and then the crucible with the sample was heated in a muffle furnace. After roasting, the roasted product was leached with water in an agitated flask while being heated by an electric hot jacket for a designed amount of time. The supernatant was vacuum- filtered and the leach residue cake was submitted to successive rinsing with water (lasting up to 10 min), before it was dried and analyzed for molybdenum and nickel contents testing.

Fig. 1 XRD pattern of roasted Ni-Mo ore
3 Results and discussion
3.1 Single sulphation roasting-water leaching experiment
As a comparison, the extraction of Mo and Ni from roasted Ni-Mo ore by single sulphation roasting and water leaching was studied. The effect of sulfuric acid addition on Mo and Ni leaching was studied under the conditions of mixing the ore with predetermined mass of sulfuric acid solution, and then roasting the mixture at 240 °C for 1 h. After roasting, the roasted product was leached by water at 95 °C for 2 h with a L/S ratio of 2 mL/g and the results are shown in Fig. 2.
Table 1 XRF elemental analysis results of roasted Ni-Mo ore (mass fraction, %)


Fig. 2 Effect of sulfuric acid addition on leaching of Mo and Ni
As can be seen, the leaching rate of Ni increased rapidly with the addition of sulfuric acid. Nevertheless, the same increase was not so clear for Mo extraction.
When the addition of sulfuric acid was 65%, the leaching rates of Mo and Ni can be achieved as 89.8% and 84.6%, respectively. Further increase of sulfuric acid resulted in insignificant increase in the leaching rates of Mo and Ni.
3.2 Single hydrochloric acid leaching
The effects of hydrochloric acid addition on Mo and Ni leaching were studied while the leaching temperature, the leaching time and the L/S ratio were kept constant as 65 °C, 30 min and 3 mL/g, respectively. The results are shown in Fig. 3.

Fig. 3 Effect of hydrochloric acid addition on leaching of Mo and Ni
According to the results illustrated in Fig. 3, the leaching rate of Mo and Ni increased quickly to 78.7% and 24.9%, respectively, with the addition of 12 mol/L hydrochloric acid at 0.219 mL/g. Further addition of hydrochloric acid resulted in insignificant increase in the leaching rates of Mo and Ni. It is obvious by comparison between Figs. 2 and 3 that when the total addition of H+ was same as 1.04 mol, the addition of sulfuric acid and hydrochloric acid was 65% and 1.0813 mL/g, respectively, the nickel leaching rate of single sulphation roasting-water leaching is much higher than that of single hydrochloric acid leaching. That is to say, single hydrochloric acid leaching cannot obtain high nickel leaching rate.
3.3 Hydrochloric acid leaching, sulphation roasting and water leaching
3.3.1 Effect of hydrochloric acid addition
It can be seen from Fig. 2 that, the sulfuric acid addition of 65% is optimal in single sulphation roasting-water leaching experiment. That is to say, 80 g roasted Ni-Mo ore needs 52 g sulfuric acid, which amounts to 1.04 mol H+. To obtain the optimum conditions of hydrochloric acid leaching and sulphation roasting, in the subsequent experiment process, the addition of total H+ was kept as 1.04 mol which was adjusted by changing the addition of hydrochloric acid and sulfuric acid. The change relationship between the addition of hydrochloric acid and sulfuric acid is given in Table 2 and the total leaching rates of Mo and Ni are shown in Fig. 4. The experiment conditions of sulphation roasting and water leaching are roasting at 240 °C for 1 h and water leaching at 95 °C for 2 h with the L/S ratio of 2 mL/g. The total leaching rate was the sum of hydrochloric acid leaching ratio and sulphation roasting- water leaching ratio.
Table 2 Addition of hydrochloric acid and sulfuric acid in 80 g roasted Ni-Mo ore


Fig. 4 Effect of hydrochloric acid addition on total leaching rates of Mo and Ni
It can be seen form Fig. 4 that the total leaching rates of both Mo and Ni increased with the increase of hydrochloric acid addition from 0 to 0.219 mL/g. Above 0.219 mL/g, the Mo leaching rates almost maintained the same while the total Ni leaching rates decreased rapidly. For subsequent experiments, the addition of hydrochloric acid in the leaching process and the addition of sulfuric acid in the sulphation roasting process were kept as 0.219 mL/g and 51.9%, respectively. Under these experimental conditions, the leaching rates of Mo and Ni can be achieved as 95.3% and 89.3%, respectively. It is indicated by comparison of Fig. 4 and Fig. 2 that, the leaching rates of Mo and Ni of the hydrochloric acid leaching followed by sulphation roasting and water leaching process are both higher than those of single sulphation roasting-water leaching process.
3.3.2 Effect of hydrochloric acid leaching temperature
Figure 5 shows the effect of hydrochloric acid leaching temperature on the leaching rates of Mo and Ni. As can be seen, the leaching rates of Mo and Ni in hydrochloric acid leaching process both increased with the increase in the leaching temperature. The leaching temperature in hydrochloric acid leaching process had no obvious effect on the leaching rate of total Mo. However, the leaching rate of total Ni increased with the leaching temperature from 25 to 65 °C, and after that, it did not increase significantly. Therefore, for subsequent experiments, the hydrochloric acid leaching temperature was kept as 65 °C.

Fig. 5 Effect of leaching temperature on Mo and Ni leaching
3.3.3 Effect of hydrochloric acid leaching time
The effect of hydrochloric acid leaching time on the leaching of Mo and Ni is shown in Fig. 6. It can be seen from Fig. 6 that the leaching rate of Mo and Ni in the hydrochloric acid leaching process and the total leaching rate both increased with the leaching time increasing from 10 to 30 min, and almost maintained constant after 30 min. Therefore, the hydrochloric acid leaching time was chosen as 30 min.
3.3.4 Effect of liquid to solid ratio (L/S)
Figure 7 shows the relationship between liquid to solid ratio (L/S) and leaching rates of Mo and Ni. As can be seen, the leaching rates of Mo and Ni in the hydrochloric acid leaching process both increased with the L/S ratio increasing from 2 to 3 mL/g, and then decreased gradually. However, the effect of L/S ratio on the total leaching rates of Mo and Ni was insignificant.
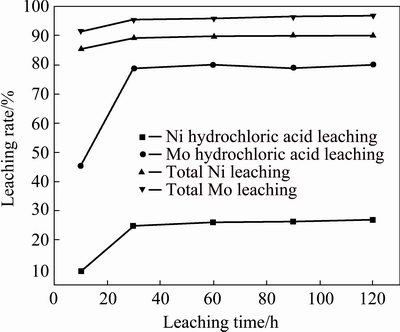
Fig. 6 Effect of leaching time on Mo and Ni leaching

Fig. 7 Effect of liquid to solid ratio on Mo and Ni leaching
3.4 Comparison of hydrochloric acid leaching and sulfuric acid leaching in first stage
As a comparison, sulfuric acid was also used as the first stage leaching reagent, and then followed by sulphation roasting and water leaching. The results of total leaching rates of Mo and Ni are shown in Fig. 8.
It was obvious by comparing hydrochloric acid leaching and sulfuric acid leaching in the first stage that when the addition of H+ was less than 0.4 mol, the Mo leaching rate is almost the same; however, the leaching rate of Ni by hydrochloric acid leaching is higher than that of sulfuric acid leaching. That is to say, the effect of hydrochloric acid as the first stage leaching reagent is better than that of sulfuric acid.
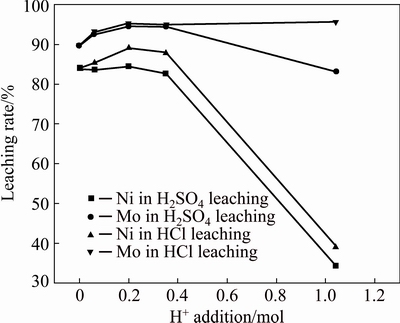
Fig. 8 Comparison of hydrochloric acid leaching and sulfuric acid leaching
3.5 Optimization of leaching condition of molybdenum and nickel
To improve the utilization of acid, the hydrochloric acid in the first stage leaching was used as the leaching solution of the calcine obtained by sulphation roasting. Figure 9 shows the process for molybdenum and nickel extraction.

Fig. 9 Schematic procedure for extraction of molybdenum and nickel from roasted Ni-Mo ore
Based on the above experiment, in hydrochloric acid leaching process, the optimum conditions would be: roasted Ni-Mo ore leached with 0.219 mL/g hydrochloric acid addition at 65 °C for 30 min with a L/S ratio of 3 mL/g. In sulphation roasting and leaching process, the optimum conditions would be: 51.9% sulfuric acid addition, roasting temperature of 240 °C for 1 h followed by leaching with hydrochloric acid leaching solution at 95 °C for 2 h. Under the optimum conditions, the leaching rates of Mo and Ni reached 95.8% and 91.3%, respectively (average of the three results). The molybdenum in leaching solution can be extracted by oxime extractant HBL101 [19]. After removing impurity, nickel can be separated and extracted by ion exchange resin Dowex M4195 [20]. In removing impurity process by adding calcium salt, sulfate radical in leaching solution can be removed in the form of calcium sulfate. Under the action of salting-out effect, chloridion can be removed in the form of sodium chloride by adding hydrochloric acid [21]. After that, waste water can be recycled.
The XRF (X-ray fluorescene) analysis of the HCl leaching residue and final leaching residue is given in Table 1. It can be seen from Table 1 that after hydrochloric acid leaching, most of Mo, As, P, Mg and V were leached into solution, and part of Ca was also leached. After sulphation roasting, most of Ni and Fe were leached into solution, and Mo also was further leached.
3.6 Phase changes during Mo and Ni extraction
To understand the process of hydrochloric leaching and sulphation roasting, XRD analysis of the hydrochloric acid leaching residue, sulphation roasted product and final residue was conducted, and the result is shown in Fig. 10.
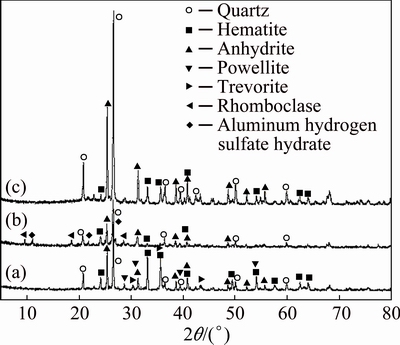
Fig. 10 XRD patterns of hydrochloric acid leaching residue (a), sulphation roasted product (b), and final residue (c)
It can be seen from Fig. 10(a) that the crystal mineral phases in hydrochloric acid leaching residue were quartz (SiO2), hematite (Fe2O3), anhydrite (CaSO4), powellite (CaMoO4) and trevorite (NiFe2O4), which were the same as the roasted Ni-Mo ore (Fig. 1). That is to say, hydrochloric acid leaching is not able to completely destroy the crystal structure of nickel and molybdenum mineral phases. Figure 10(b) shows that the powellite (CaMoO4) and trevorite (NiFe2O4) phases disappeared after the sulphation roasting. By comparing Figs. 10(b) and (a), it was found that two new phases rhomboclase (FeH(SO4)2(H2O)4) and aluminum hydrogen sulfate hydrate (AlH(SO4)2H2O) formed during sulphating roasting. As can be seen form Fig. 10(c) that the peaks of rhomboclase and aluminum hydrogen sulfate hydrate disappeared due to their dissolution during the leaching process. In the final residue, there were only three crystalline mineral phases, namely, quartz (SiO2), anhydrite (CaSO4) and hematite (Fe2O3). From Table 1 and Fig. 10, it can be seen that for nickel extraction, it is important to destroy the crystal structure of trevorite (NiFe2O4), which can only be decomposed by sulphation roasting.

Fig. 11 SEM images of Ni-Mo ore
3.7 Surface morphology changes
Figure 11 shows the morphologies of the raw roasted Ni-Mo ore, hydrochloric acid leach residue, sulphation roasted product and final residue. As can be seen from Fig. 11(b), after hydrochloric acid leaching, the particles became finer, and the leaching residue accounted for 76.4% of the roasted Ni-Mo ore. After sulphation roasting, the particles aggregated and bonded together. Finally, the bonded particles became loose again after water leaching. It was obvious by comparing Figs. 11(a) and (d) that, the particles of final residue became finer and smaller. The final residue accounted for 50.3% of the roasted Ni-Mo ore.
4 Conclusions
1) The technique of hydrochloric acid leaching- sulphation roasting followed by leaching with the first stage hydrochloric acid leaching solution can get higher leaching rates of Mo and Ni than single sulphation roasting-water leaching.
2) Under the optimum conditions of hydrochloric acid leaching (roasted Ni-Mo ore leached with 0.219 mL/g hydrochloric acid addition at 65 °C for 30 min with a L/S ratio of 3 mL/g), sulphation roasting (51.9% sulfuric acid addition, roasting temperature 240 °C for 1 h), followed by leaching with the first stage hydrochloric acid leaching solution at 95 °C for 2 h, the leaching rates of Mo and Ni reached 95.8% and 91.3%, respectively.
References
[1] PENG J, WANG X W, JIANG C J, WANG M Y, MA Y Q, XIANG X Y. Separation of Mo(VI) and Fe(III) from the acid leaching solution of carbonaceous Ni-Mo ore by ion exchange [J]. Hydrometallurgy, 2014, 142: 116-120.
[2] FAN D L, ZHANG T, JIE Y, PAAVA J, KRIBEK B, DOBES P, VARRIN I, ZAK K. Geochemistry and origin of tin-polymetallic sulfide deposits hosted by the Devonian black shale series near Dachang, Guangxi, China [J]. Ore Geology Reviews, 2004, 241(1-2): 103-120. (in Chinese)
[3] WANG Ming-yu, WANG Xue-wen, JIANG Chang-jun, MA Yi-qian, FAN Ye-ye, XIANG Xiao-yan. Comprehensive utilization process and research status of Ni-Mo ore [J]. Chinese Journal of Rare Metals, 2012, 36(2): 321-328. (in Chinese)
[4] HU Hui-ying, ZHAO Qing-lei, ZHANG Zhi-hua, ZHANG Zhi-xiong. The research current situation of the treatment process for Ni-Mo ores [J]. Metal Materials and Metallurgy Engineering, 2013,41(3): 42-46. (in Chinese)
[5] SHI L H, WANG X W, WANG M Y, PENG J, XIAO C X. Extraction of molybdenum from high-impurity ferromolybdenum by roasting with Na2CO3 and CaO and leaching with water [J]. Hydrometallurgy, 2011, 108: 214-219.
[6] LI Qing-gang, XIAO Lian-sheng, ZHANG Gui-qing, ZHANG Qi-xiu. Process and practice of ammonium molybdate production from Ni Mo ore by hydrometallurgy [J]. Chinese Journal of Rare Metals, 2007, (31): 85-89. (in Chinese)
[7] WANG M S, WEI C, FAN G, LI M T, DENG Z G, WANG S F. Selective extraction of Mo from a Ni-Mo ore using pressure alkaline leaching [J]. Hydrometallurgy, 2015, 153: 6-11.
[8] PENG Jian-rong, YANG Da-jin, CHEN Jia-xi, YAN Jiang-feng. Experimental study on alkaline leaching of crude molybdenite under pressure of oxygen [J]. Chinese Journal of Rare Metals, 2007, 31: 110-113. (in Chinese)
[9] WANG M Y, WANG X W, LIU W L. A novel technology of molybdenum extraction from low grade Ni-Mo ore [J]. Hydrometallurgy, 2009, 97: 126-130.
[10] ZHAO Zhong-wei, XU Xiao-yang, CHEN Xing-yu, HUO Guang-sheng, CHEN Ai-liang, LIU Xu-heng, XU Hui. Thermodynamics and kinetics of adsorption of molybdenum blue with D301 ion exchange resin [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 686-692.
[11] ZHAO Z W, LI J T, CAO C F, HUO G S, ZHANG G, LI H G. Recovery and purification of molybdenum from Ni-Mo ore by direct air oxidation in alkaline solution [J]. Hydrometallurgy, 2010, 103: 68-73.
[12] LIU W P, XU H, YANG X Y, SHI X C. Extraction of molybdenum from low-grade Ni-Mo ore in sodium hypochlorite solution under mechanical activation [J]. Mineral Engineering, 2011, 24: 1580-1585.
[13] WANG Si-fu, WEI Chang, DENG Zhi-gan, LICun-xiong, LI Xin-bing, WU Jun, WANG Ming-shuang, ZHANG Fan. Extraction of molybdenum and nickel from Ni-Mo ore by pressure acid leaching [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3083-3088.
[14] XIAO Chao-long, XIAO Lian-sheng, GONG Bo-fan, GUO Chao. Study on hydrometallurgical leaching of Ni-Mo ore [J]. Rare Metals and Cemented Carbides, 2010, 38(4): 1-4. (in Chinese)
[15] BAO Zheng-xiang, WAN Rong-jiang, BAO Jue-min. Metallogenic characteristics and genesis of the Ni-Mo deposits in north-western Hunan [J]. Hubei Geology & Mineral Resources, 2001, 15(1): 14-21. (in Chinese)
[16] WANG X W, PENG J, WANG M Y, YE P H,XIAO Y. The role of CaO in the extraction of Ni and Mo from carbonaceous shale by calcification roasting, sulphation roasting and water leaching [J]. International Journal of Mineral Processing, 2011, 100: 130-135.
[17] WANG M Y, WANG X W. Extraction of molybdenum and nickel from carbonaceous shale by oxidation roasting, sulphation roasting and water leaching [J]. Hydrometallurgy, 2010, 102: 50-54.
[18] LIN Da-yi, ZHANG Yong-de, WU Min. Non-ferrous metal ore and mineral smelting and product analysis [M]. Beijing: Metallurgical Industry Press, 2007: 25-28. (in Chinese)
[19] ZENG L, LIAO X L, SUN Y H, XIAO L S. Direct extraction of molybdenum from high acid leach solutions of Ni-Mo ore using an oxime extractant of HBL101 [J]. Int Journal of Refractory Metals and Hard Materials, 2015, 51: 14-18.
[20] XIAO Chao-long. The study of treating nickel-molybdenum ore using hydrometallurgical process [D]. Changsha: Central South University Press, 2011. (in Chinese)
[21] MA Y Q, WANG X W, WANG M Y, JIANG C J, XIANG X Y, ZHANG X L. Separation of V(IV) and Fe(III) from the acid leach solution of stone coal by D2EHPA/TBP [J]. Hydrometallurgy, 2015, 153: 38-45.
羡鹏飞,周升帆,王明玉,王学文,陈边防
中南大学 冶金与环境学院,长沙 410083
摘 要:为了从焙烧的镍钼矿中提取钼和镍,研究了盐酸浸出-硫酸化焙烧和水浸出处理焙烧后镍钼矿的过程。实验结果表明,焙烧的镍钼矿经过盐酸浸出-硫酸化焙烧然后水浸出后能够获得高的钼和镍浸出率。氧化焙烧的镍钼矿添加0.219 mL/g盐酸(12 mol/L)在液固比为3 mL/g的条件下于65 °C浸出30 min;浸出渣添加51.9%浓硫酸在240 °C下焙烧1 h;焙砂物料采用已经获得的第一段盐酸浸出液在95 °C浸出2 h,钼和镍的总浸出率分别达到95.8%和91.3%。
关键词:钼;镍;镍钼矿;盐酸浸出;硫酸化焙烧;水浸出
(Edited by Xiang-qun LI)
Foundation item: Project (51104186) supported by the National Natural Science Foundation of China; Projects (2016zzts282, 2016zzts283) supported by the Fundamental Research Funds for the Central Universities of Central South University, China
Corresponding author: Ming-yu WANG; Tel: +86-731-88830247; E-mail: wmydxx@163.com
DOI: 10.1016/S1003-6326(17)60025-6

