Trans. Nonferrous Met. Soc. China 24(2014) 1989-1994
Electrochemical corrosion behavior of bulk ultra-fine grained Fe-Ni-Cr alloy
Zhan-wen WU1,2, Ji CHEN1, Nan PIAO1, Cheng SUN3, W. HASSAN1, Xin-hang ZHANG3, Yu-jun XIE1
1. Department of Mechanical Engineering, Liaoning Shihua University, Fushun 113001, China;
2. CNOOC Energy Technology and Services–Pipe Engineering Co., Tianjin 300452, China;
3. Department of Mechanical Engineering, Materials Science and Engineering Program, Texas A&M University, College Station, Texas 77843-3123, USA
Received 20 May 2013; accepted 13 March 2014
Abstract: The corrosion behavior of bulk ultra-fine grained (UFG) Fe-Ni-Cr alloy prepared by equal-channel angular pressing technique was investigated in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution by electrochemical measurements. As compared to the coarse grained (CG) counterpart, the UFG alloy exhibits an acceleration of the active dissolution and a shrunk passive region with a higher passive current. The Mott-Schottky analysis in conjunction with the point defect model indicates that the donor diffusion coefficient in the passive films of the UFG sample increases greatly to one magnitude order higher and the donor density is slightly lower than that of the CG sample.
Key words: stainless steel; Fe-Ni-Cr; polarization; acid corrosion; passivation
1 Introduction
The application of stainless steel (SS) materials is limited due to some undesirable properties such as relatively low wear ability and low strength. The equal channel angular pressing (ECAP) is one of the potential techniques by employing grain refinement to improve mechanical properties of bulk materials [1]. The effects of ultra-fine grained (UFG) microstructure formed by ECAP technique on mechanical properties [2-7], phase transformation characteristics [8], thermal stability [9] and radiation tolerance [10] of SS materials have been extensively investigated.
However, not many investigations have been reported on the corrosion behavior of bulk as-ECAPed SS material, especially its passive properties. Extensive studies on the corrosion resistance of SS by surface nanocrystallisation processes have been made [11-17]. YE et al [11] found that the localized corrosion resistance of the 309 SS in chloride ion-containing solution was enhanced greatly due to the formation of a compact and more stable passive film on the nanocrystalline coating. MENG et al [12] found that the nanocrystallization by magnetron sputtering accelerated the active dissolution of Fe-10Cr coating but improved chemical stability of the passive film. LIU et al [13] found that the grain size of the nanocrystalline SS coating was beneficial to the repassivation and decreasing the probability of stable pits formation due to the formation and continuous growth of nano-scale oxide particles. LIU et al [14] also found that the pitting corrosion resistance of the nanocrystalline Ni-based supperalloy coating increased significantly with decreasing of grain size in acidic solution, which was attributed to the smaller grain size promoting the formation of compact passive film. YE et al [15] reported enhanced corrosion resistance of the 309 SS coating in the transpassive region in Na2SO4 solution. They attributed the enhanced corrosion resistance to the homogeneous distribution of Cr in passive film and decreased the carrier density of the oxide film, which reduced the dissolution rate of the oxide film. Above studies have demonstrated that grain size of nanocrystalline SS with far fewer dislocations improves passive properties, which enhances corrosion resistance of SS compared with that of conventional coarse grained alloys.
It should be noted that the effect of refining processes on the corrosion resistance of SS depends on not only grain size, but also the density of dislocations. But only few studies have focused on electrochemical behavior of SS materials with nanocrystalline structure containing high-density dislocations [18,19]. WANG and LI [18] reported that nanocrystallization by sandblasting without annealing weakened the mechanical property of the passive film on 304 SS surface resulting from the poor interfacial bond between the film and the substrate containing large number of dislocations and non-equilibrium grain boundaries. ZHENG et al [19] attributed an improved corrosion resistance of the ECAPed 304 SS (with a grain size of 80-120 nm) in dilute H2SO4 solution to the improved compactness and stability of passive film. The above studies have deepened the understanding of corrosion behavior of SS with nanocrystalline structure containing high-density disloactions, but arguments exists about their corrosion mechanism. Therefore, it is deserved to further investigate the corrosion behavior of SS materials with nanocrystalline structure containing high-density disloactions.
In this work, a bulk UFG Fe-Ni-Cr alloy with high-density dislocations was prepared by ECAP technique and the electrochemical behavior of the as-ECAPed sample in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution were investigated in comparison with that of the as-cast CG counterpart by potentiodynamic polarization. The effect of UFG microstructure on the formation and properties of passive film was discussed by Mott–Schottky analysis in conjunction with the point defect model.
2 Experimental
The raw Fe-Ni-Cr alloy with average grain size of 700 μm was prepared by vacuum cast and subsequent hot isostatic pressing. The composition of this alloy is listed in Table 1. A total of four ECAP passes with route Bc was performed at 500 °C to refine the microstructure of the alloy. Route Bc means that between each subsequent pass, the sample was rotated by 90° around its longitudinal axes. The detailed technical parameters of the sample preparation can be found elsewhere [9]. Both of the as-cast CG and the as-ECAPed UFG alloys were cut into blocks with the dimension of 15 mm×15 mm×5 mm. The samples were embedded in epoxy resin, leaving a working square area of 1.5 cm×1.5 cm uncovered for performing the electrochemical experiments. The exposed surface was successively ground to 2000-grit using SiC abrasive paper, rinsed with ethanol in an ultrasonic cleaner and then dried in air.
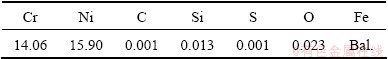
Table 1 Chemical composition of as-cast Fe-Ni-Cr alloy (mass fraction, %)
Experiment was performed on a JEOL 2010 transmission electron microscope operated at 200 kV and equipped with a Gatan SC1000 ORIUS CCD camera.
The electrochemical experiments were performed in a conventional three-electrode cell using EG&G PARSTAT2273 electrochemical workstation. The counter electrode was a graphite sheet and all the potentials were measured versus the saturated calomel electrode (SCE). The working electrode was the Fe-Ni-Cr alloy sample. A potential scanning rate of 0.5 mV/s was applied in the potentiodynamic polarization tests. For the Mott- Schottky relationship measurements, a 5 mV amplitude sine wave modulated signal was employed with a frequency of 1 kHz and a step rate of 20 mV. Each measurement was performed at least 3 times for data reproducibility. Prior to all experiments, the working electrodes were cathodically pre-polarized at -1 V for 120 s to remove the surface oxide films formed in air. All experiments were performed at 25 °C water bathing in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution. All the reagents were analytically pure and all aqueous solutions were prepared using distilled water.
3 Results
3.1 Microstructure of as-ECAPed Fe-Ni-Cr alloy
Figures 1(a) and (b) show the bright-field and the corresponding dark-field TEM micrographs of the as-ECAPed Fe-Ni-Cr alloy after 4 passes. Obvious fragmentization could be observed, resulting in the formation of large amount of equiaxed grains with non-equilibrium grain boundaries (GBs) accumulated with high density of dislocations. The inserted selected area electron diffraction (SAED) pattern in Fig. 1(a) shows the discontinuous diffraction rings, indicating that the grains have been refined greatly and the phase is still austenite. Statistics of grain size in the as-ECAPed Fe-Ni-Cr alloy sample, as shown in Fig. 1(c), reveal the UFG microstructure with an average grain size of (215±25) nm with a narrow distribution. The XRD analysis indicates that the dislocation density increases from ~1010 to ~1014 m-2 after ECAP processing.
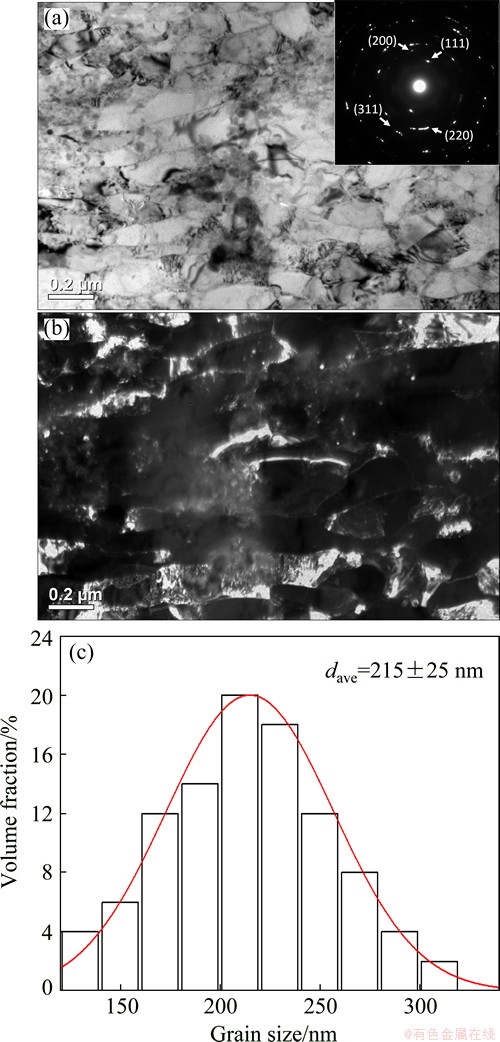
Fig. 1 Bright-field TEM micrograph and corresponding SAED pattern of as-ECAPed Fe-Ni-Cr alloy (a), corresponding dark-field TEM micrograph (b) and statistics of grain size distribution (c)
3.2 Potentiodynamic polarization curves
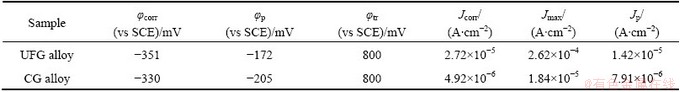
Figure 2 shows the potentiodynamic polarization curves of the as-ECAPed UFG Fe-Ni-Cr alloy with that of the as-cast CG counterpart in 0.25 mol/L Na2SO4 + 0.05 mol/L H2SO4 solution. The electrochemical parameters derived from the polarization curves are listed in Table 2, where φcorr, φp and φtr represent the free corrosion potential, passive potential and film breakdown potential, respectively; and Jcorr, Jmax and Jp are the free corrosion current density, critical current density and passive current density, respectively. The value of Jcorr is calculated by extrapolating the cathode Tafel lines back to the corresponding φcorr. The UFG Fe-Cr-Ni alloy has φcorr and Jcorr values of about -351 mV (vs SCE) and 2.72×10-5 A/cm2, respectively, in contrast with those of about -330 mV and 4.92×10-6 A/cm2 for the CG counterpart. The UFG Fe-Cr-Ni alloy exhibits a much higher Jmax value, a higher φp value and a shrunk passive region with an increasing Jp value to about 1.42×10-5 A/cm2 as compared with the CG counterpart, indicating that UFG structure weakens the passivation ability of Fe-Cr-Ni alloy.
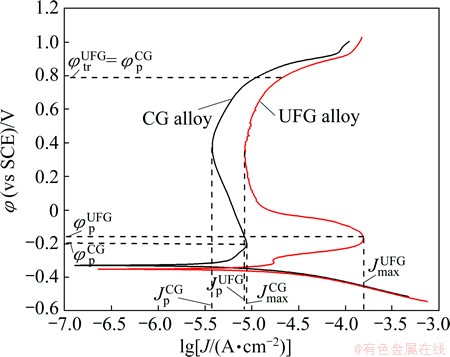
Fig. 2 Potentiodynamic polarization curves for UFG Fe-Ni-Cr alloy and CG counterpart in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution
4 Discussion
4.1 Effect of UFG microstructure on active dissolution
The UFG Fe-Ni-Cr alloy displays a higher Jcorr value and a lower φcorr value than those of the CG alloy, indicating that the dissolution in the active region is obviously accelerated for the UFG alloy as compared to the CG counterpart. The UFG materials are characterized by a large fraction of non-equilibrium grain boundaries with highly excess stored energy [12], which tend to be the active points for the acceleration of the active dissolution.
Table 2 Electrochemical parameters derived from potentiodynamic polarization curves for UFG Fe-Ni-Cr alloy and CG counterpart
4.2 Effect of UFG microstructure on semiconducting property of passive film
Generally, the corrosion resistance of a passive alloy is related to the semiconducting properties of a passive film on the surface, which are determined by concentration and diffusivity of charge carriers such as the point defect including donor and/or acceptor. According to the conventional Mott-Schottky space-charge theory [20], as the space-charge of the passive film is under the depletion condition, a linear relationship is expected between reciprocal of the space-charge capacitance square (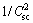 ) and the applied potential (φ) for the film, which can be described by the equations for different types of semiconductors as follows:
) and the applied potential (φ) for the film, which can be described by the equations for different types of semiconductors as follows:
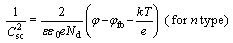 (1)
(1)
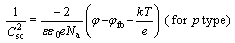 (2)
(2)
where Nd and Na are the charge carrier densities of the donor and the acceptor, respectively; ε is the dielectric constant of the passive film (here ε is taken as 15.6 [21]); ε0 is the vacuum permittivity; e is the elementary charge; k is the Boltzman constant; T is the absolute temperature and φfb is the flat band potential.
A nearly pure capacitive frequency response could be expected for the electrochemical impedance spectroscopy measured at frequencies higher than 1 kHz [22]. The resulting steady-state film thickness of the passive film Lss could be estimated as
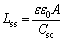 (3)
(3)
where A is the surface area of the sample.
The electric field strength of the passive film εL could be extracted from a linear relationship between the steady-state film thickness Lss and the film formation potential φf as given in Ref. [23]:
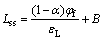 (4)
(4)
where α is the polarizability of the passive film/solution interface (α is taken as 0.45 [24]) and B is a constant.
According to the point defect model (PDM) [25], the charge carrier densities of the donor Nd is related to the film formation potential φf with an equation expressed as
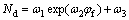 (5)
(5)
where ω1, ω2 and ω3 are unknown fitting constants. By taking the fitted ω3 and εL values, the diffusivity of the donor defect in passive film, Dd, can be calculated as
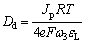 (6)
(6)
where R is the universal gas constant; F is the Farady constant.
Figure 3 shows the comparison of the Mott-Schottky plots of the passive films formed at different potentials (φf) on the UFG Fe-Ni-Cr alloy and the CG sample, respectively. The data in the scanning range from 0.3 to 0.6 V could be roughly fitted by a linear relationship with positive slopes, indicating that both of the passive films formed on the UFG Fe-Ni-Cr alloy and the CG counterpart are n-type. The calculated donor density, Nd, at different formation potentials are in the range from 6.31×1021 to 9.82×1021 cm-3 for the UFG alloy and in the range from 1.01×1022 to 1.93×1022 cm-3 for the CG alloy, respectively. The lower donor density at the metal/film interface on the UFG sample implied that the passive current Jp of UFG alloy should be lower than that of CG counterpart, which is different from our observation of higher Jp of UFG alloy in potentiodynamic polarization curves. It indicates that the point defect density of the passive films contributes little to the corrosion behavior of UFG alloy.
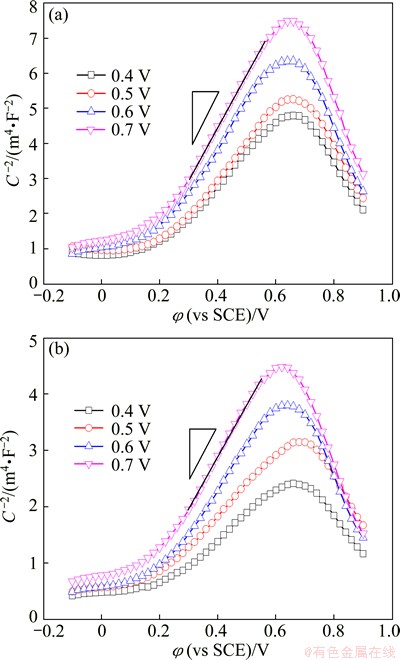
Fig. 3 Mott-Schottky plots of UFG Fe-Ni-Cr alloy (a) and CG counterpart (b) in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution
Figure 4 shows the comparison of the point defect densities of the passive films formed on the UFG Fe-Ni-Cr alloy and the CG counterpart as a function of formation potential in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution. Both Nd of the passive films decrease exponentially with the increase of formation potential, and are fitted using Eq. (5) as
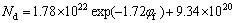
(for UFG alloy) (7)
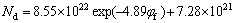
(for CG alloy) (8)
The values of ω3 are directly extracted as 9.34×1020 for the UFG Fe-Ni-Cr alloy and 7.28×1021 for the CG counterpart, respectively.
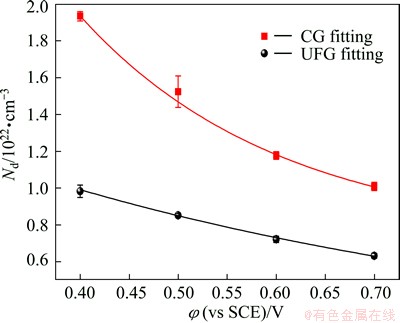
Fig. 4 Relationships between point defect densities of passive films formed on UFG Fe-Ni-Cr alloy (a) and CG counterpart (b) and formation potential in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution
Figure 5 shows the steady-state thickness of the passive film formed on UFG Fe-Ni-Cr alloy and CG counterpart as a function of formation potential in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution. Steady-state passive films formed on UFG Fe-Ni-Cr alloy and CG counterpart increase with the increase of formation potentials as a linear function. The electric fields εL extracted from Eq. (4) are 5.98×106 V/cm for the UFG alloy and 5.61×106 V/cm for the CG alloy, respectively, which is in good agreement with the ~106 V/cm normally found for passive film formation on SS at near ambient temperature [26]. Taken the fitted ω3 and ε into Eq. (6), the diffusivities of the point defect in the passive films are 1.02×10-16 cm2/s for the UFG Fe-Ni-Cr sample and 7.76×10-18 cm2/s for the CG counterpart, respectively. This agrees with the potentiodynamic polarization curves observation that the Jp of UFG alloy is much lower than that of CG counterpart.
Previous investigations in some nanocrystalline alloys using magnetron sputtering and electrodepositing [11-17] have shown that the grain size reduction can decrease both the donor density and the diffusion coefficient, resulting in the formation of more homogeneous and protective passive film. As a contrast, the bulk UFG Fe-Ni-Cr alloy exhibits a slightly lower donor density but a much increased diffusion coefficient, which may be due to the large amount of nonequilibrium GBs together with high density of dislocations (~1014 m-2), resulting in a poor interfacial bonding between the passive film and the substrate as suggested in Ref. [18]. The stoichiometry and microstructure of the passive film formed on the UFG Fe-Ni-Cr alloy will be further investigated.
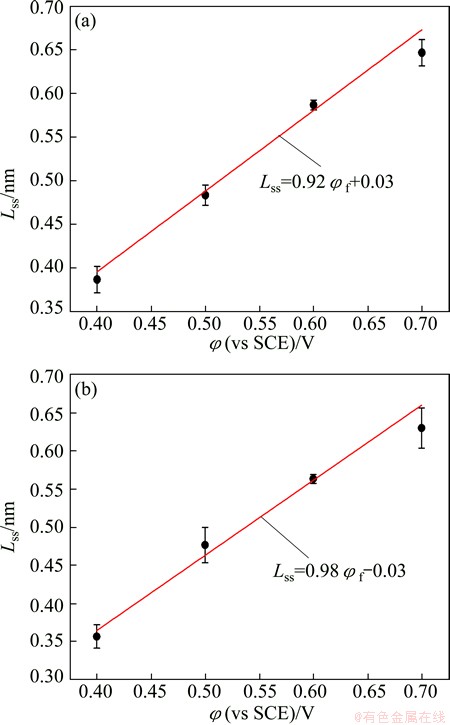
Fig. 5 Steady film thickness of passive films formed on UFG Fe-Ni-Cr alloy (a) and CG counterpart (b) as function of formation potential in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution
5 Conclusions
1) Bulk UFG Fe-Ni-Cr austenite alloy samples with an grain size of (215±25) nm were prepared by ECAP technique performed at 500 °C after four passes.
2) The UFG alloy has an accelerated active dissolution and decreased ability to passivation in 0.25 mol/L Na2SO4+0.05 mol/L H2SO4 solution as compared to the CG counterpart.
3) The Mott-Schottky analysis together with the point defect model indicates that the donor density at the metal/film interface of the UFG sample is slightly lower than that of the CG sample, but the donor diffusion coefficient in the passive films is increased greatly to one magnitude order higher.
References
[1] VALIEV R Z, ISLAMGALIEV R K, ALEXANDROV I V. Bulk nanostructured materials from severe plastic deformation [J]. Progress in Materials Science, 2000, 45: 103-189.
[2] YANG M X, YANG G, LIU Z D, DU X Q, HUANG X C. Microstructure and mechanical properties of 0Cr13 ferritic stainless steel processed by equal-channel angular pressing and subsequent annealing treatment [J]. Acta Metallurgica Sinica, 2012, 48: 1422-1430. (in Chinese)
[3] QU S, HUANG C X, GAO Y L, YANG G, WU S D, ZANG Q S, ZHANG Z F. Tensile and compressive properties of AISI 304L stainless steel subjected to equal channel angular pressing [J]. Material Science and Engineering A, 2008, 475: 207-216.
[4] HUANG C X, YANG G, GAO Y L, WU S D, ZHANG Z F. Influence of processing temperature on the microstructures and tensile properties of 304L stainless steel by ECAP [J]. Material Science and Engineering A, 2008, 485: 643-650.
[5] HAEL M, HEINZ W H. Cyclic deformation and fatigue properties of very fine-grained metals and alloys [J]. International Journal of Fatigue, 2010, 32: 1413-1427.
[6] UENO H, KAKIHATA K, KANEKO Y, HASHIMOTO S, VINOGRADOV A. Enhanced fatigue properties of nanostructured austenitic SUS 316L stainless steel [J]. Acta Materialia, 2011, 59: 7060-7069.
[7] CHEN L, YUAN F P, JIANG P, WU X L. Mechanical properties and nanostructures in a duplex stainless steel subjected to equal channel angular pressing [J]. Material Science and Engineering A, 2012, 551: 154-159.
[8] YANG G, HUANG C X, WU S D, ZHANG Z F. Strain-induced martensitic transformation 304L austenitic stainless steel under ECAP deformation [J]. Acta Metallurgica Sinica, 2009, 45: 906-911. (in Chinese)
[9] SUN C, YANG Y, LIU Y, HARTWIG K T, WANG H, MALOY S A, ALLEN T R, ZHANG X. Thermal stability of ultrafine grained Fe-Cr-Ni alloy [J]. Material Science and Engineering A, 2012, 542: 64-70.
[10] SUN C, YU K Y, LEE J H, LIU Y, WANG H, SHAO L, MALOY S A, HARTWIG K T, ZHANG X. Enhanced radiation tolerance of ultrafine grained Fe–Cr–Ni alloy [J]. Journal of Nuclear Materials, 2012, 420: 235-240.
[11] YE W, LI Y, WANG F H. Effects of nanocrystallization on the corrosion behavior of 309 stainless steel [J]. Electrochimica Acta, 2006, 51: 4426-4432.
[12] MENG G Z, LI Y, WANG F H. The corrosion behavior of Fe-10Cr nanocrystalline coating [J]. Electrochimica Acta, 2006, 51: 4277-4284.
[13] LIU L, LI Y, WANG F H. Influence of nanocrystallization on pitting corrosion behavior of an austenitic stainless steel by stochastic approach and in situ AFM analysis [J]. Electrochimica Acta, 2010, 55: 2430-2436.
[14] LIU L, LI Y, WANG F H. Influence of grain size on the corrosion behavior of a Ni-based superalloy nanocrystalline coating in NaCl acidic solution [J]. Electrochimica Acta, 2008, 53: 2453-2462.
[15] YE W, LI Y, WANG F H. The improvement of the corrosion resistance of 309 stainless steel in the transpassive region by nano-crystallization [J]. ElectrochimicaActa, 2009, 54: 1339-1349.
[16] PENG X, YAN J, ZHOU Y, WANG F. Effect of grain refinement on the resistance of 304 stainless steel to breakaway oxidation in wet air [J]. Acta Materialia, 2005, 53: 5079-5088.
[17] PAN C, LIU L, LI Y, WANG S G, WANG F H. Passive film growth mechanism of nanocrystalline 304 stainless steel prepared by magnetron sputtering and deep rolling techniques [J]. Electrochimica Acta, 2011, 56: 7740-7748.
[18] WANG X Y, LI D Y. Mechanical and electrochemical behavior of nanocrystalline surface of 304 stainless steel [J]. Electrochimica Acta, 2002, 47: 3939-3947.
[19] ZHENG Z J, GAO Y, GUI Y, ZHU M. Corrosion behaviour of nanocrystalline 304 stainless steel prepared by equal channel angular pressing [J]. Corrosion Science, 2012, 54: 60-67.
[20] GARRETT C G B, BRATTAIN W H. Physical theory of semiconductor surfaces [J]. Physical Review, 1955, 99: 376-387.
[21] SIMOS A M P, FERRIRA M G S, RONDOT B, BELO M C. Study of passive films formed on AISI 304 stainless steel by impedance measurements and photoelectrochemistry [J]. Journal of Electrochemical Society, 1990, 137: 82-87.
[22] NICIC I, MACDONALD D D. The passivity of Type 316L stainless steel in borate buffer solution [J]. Journal of Nuclear Materials, 2008, 379: 54-58.
[23]  I. Diffusivity of anion vacancies in WO3 passive films [J]. Electrochimica Acta, 2007, 52: 6771-6777.
I. Diffusivity of anion vacancies in WO3 passive films [J]. Electrochimica Acta, 2007, 52: 6771-6777.
[24] MACDONALD D D, MACDONALD M U. Theory of steady-state passive films [J]. Journal of Electrochemical Society, 1990, 137: 2395-2402.
[25] SIKORA E, SIKORA J, MACDONALD D D. A new method for estimating the diffusivities of vacancies in passive films [J]. Electrochimica Acta, 1996, 41: 783-789.
[26] FATTAH-ALHOSSEINI A, SAATCHI A, GOLOZAR M A, RAEISSI K. The passivity of AISI 316L stainless steel in 0.05 M H2SO4 [J]. Journal of Applied Electrochemisty, 2010, 40: 457-461.
块体超细晶Fe-Ni-Cr合金的腐蚀行为
武占文1,2,陈 吉1,朴 楠1,孙 成3,W. HASSAN 1,张星航3,谢禹钧1
1. 辽宁石油化工大学 机械工程学院,抚顺 113001;2. 中海油能源发展股份有限公司 管道工程分公司,天津 300452;
3. Department of Mechanical Engineering, Materials Science and Engineering Program, Texas A&M University, College Station, Texas 77843-3123, USA
摘 要:利用等通道转角挤压方法制备块体超细晶Fe-Ni-Cr合金,对其在0.25 mol/L Na2SO4+0.05 mol/L H2SO4溶液中的耐蚀性进行研究。与粗晶合金相比,超细晶合金表现出加速的活性溶解过程,钝化区间缩小,维钝电流密度更大。对表面钝化膜进行Mott-Schottky曲线测试并结合点缺陷模型分析,结果表明,超细晶合金钝化膜内载流子的扩散系数较粗晶合金显著提高一个数量级,载流子密度略有降低。
关键词:不锈钢;Fe-Ni-Cr合金;极化;酸性腐蚀;钝化
(Edited by Chao WANG)
Foundation item: Project (201202127) support by Liaoning Provincial Natural Science Foundation of China under Grant; Project (LJQ2011033) support by Program for Liaoning Excellent Talents in University
Corresponding author: Ji CHEN; Tel: +86-13516005504; E-mail: jchen_Lsu@hotmail.com
DOI: 10.1016/S1003-6326(14)63280-5