DOI: 10.11817/j.issn.1672-7207.2017.12.002
氧化钼/CPC界面吸附层结构对浮选行为的影响
王振,徐龙华,肖军辉,王进明,傅开彬,张海阳
(西南科技大学 固体废物处理与资源化教育部重点实验室,四川 绵阳,621010)
摘要:通过Zeta电位测试、吸附量测量、荧光探针及单矿物浮选研究氯化十六烷基吡啶(CPC)在氧化钼表面吸附过程及吸附层结构和其对矿物浮选行为的影响。研究结果表明:氧化钼在水溶液中的等电点为1.9,在pH 2~12之内荷负电,吡啶阳离子依靠静电作用及疏水缔合作用吸附;CPC的临界胶束浓度(CMC)为9.6×10-4 mol/L,吡啶阳离子与氧化钼表面的吸附作用符合“四区域模型”,在吡啶捕收剂浓度低于临界半胶束浓度(CHC,约6.4×10-4 mol/L)时,主要靠静电作用吸附,之后碳链间的疏水缔合起主要作用,且CPC浓度在CHC附近时矿物表面疏水性最强。当吡啶捕收剂浓度过大时确实会导致矿物浮选回收率的下降,临界浓度十分接近吡啶捕收剂CHC。
关键词:氧化钼;吡啶捕收剂;吸附;表面微极性;浮选
中图分类号:TD913 文献标志码:A 文章编号:1672-7207(2017)12-3147-05
Influence of adsorbed layer structure of CPC/molybdite interface on flotation
WANG Zhen, XU Longhua, XIAO Junhui, WANG Jinming, FU Kaibin, ZHANG Haiyang
(Key Laboratory of Solid Waste Treatment and Resource Recycle Ministry of Education,
Southwest University of Science and Technology, Mianyang 621010, China)
Abstract: The adsorbed layer structure of CPC/molybdite interface and its effect on flotation were studied through Zeta potential measurements, adsorbed amount tests, fluorescence probe and micro-flotation. The results show that the IEP of molybdite is 1.9 and it is negative charged in pH range of 2-12. The adsorption of CPC depends on molybdite by electrostatic force and hydrophobic force. A CMC value of 9.6×10-4 mol/L is obtained by adsorption isotherm, and the four region model is suitable for the adsorption of CPC on molybdite. When the CPC concentration is below CHC, the electrostatic force is the reason for adsorption between CPC and molybdite, while hydrophobic force is responsible for the adsorption at the CPC concentration over CHC. The greatest hydrophobicity is achieved around CHC. Mineral recovery declines when the collector concentration surpasses its CHC.
Key words: molybdite; pyridine; adsorption; micropolarity; flotation
钼是一种人体及动植物必需的微量元素,其在汽车工业、电子行业及国防军工中均有重要应用[1]。一直以来,硫化钼矿石是钼金属的主要来源,然而随着硫化钼资源储量的日益下降,钼行业面临越来越突出的资源问题[2-4]。因此,高效利用氧化钼矿石变得愈加重要。氧化钼矿物主要有钼钙矿、钼华、彩钼铅矿等,它们的可浮性均较差,采用浸出的方法从氧化钼矿石中提取钼元素,然后制取钼酸铵是一种常规方法[5]。但是由于所处理的氧化钼矿石品位低、成分复杂,在浸出的过程中会消耗大量的浸出剂,能耗也很高,这主要是因为矿石中存在大量的脉石矿物,大部分的药剂被脉石矿物颗粒表面吸附[6-8]。因此,通过选矿的方法预先富集含钼矿物,浸出处理含较少脉石矿物的粗精矿将会大大减少药剂耗量,提高流程经济性[9]。如果能够加以利用氧化钼矿物,将会一定程度上弥补钼资源的缺口。但是,氧化钼的表面亲水性较强[10],不具有天然可浮性,在以往的研究中研究较少。在前期研究中,发现吡啶类捕收剂对氧化钼具有一定捕收能力,有潜力在氧化钼矿石的浮选法处理中作为氧化钼的捕收剂[3]。吡啶类捕收剂对矿物的捕收作用主要依靠其吡啶环头基中的氮原子作为亲固原子进行吸附,其作用方式类似阳离子胺类捕收剂,因此在浮选捕收剂的分类中通常归为胺类捕收剂。据报道,阳离子捕收剂具有矿浆适应性好、用量少及浮选精矿含水量小等优点[11],关于阳离子捕收剂与硅酸盐类矿物的研究有很多报道,但是对于吡啶类阳离子捕收剂与金属氧化物之间的作用鲜有报道。基于此,本文作者以氧化钼和氯化十六烷基吡啶为研究对象,从矿物/药剂界面吸附入手,研究吡啶捕收剂对氧化钼的浮选机理,并深入研究了界面吸附层结构对矿物浮选行为的影响。
1 研究方法
1.1 试验矿样与药剂
本试验所用的氧化钼矿物(MoO3,纯度为99.6%)购自北京水远山长矿物标本公司。试验矿样分别经过瓷球罐磨碎、筛分,粒级小于0.076 mm的用于浮选试验,粒级小于0.038 mm的用于吸附量测定。
试验采用分析纯氯化十六烷基吡啶作为矿物捕收剂,分析纯试剂NaOH和HCl作为矿浆pH调整剂,分析纯芘用于荧光探针试验,所有用水为去离子超纯水。
1.2 动电位测量
采用美国 BECKMAN COULTER 公司的Coulter Delsa 440sx型Zeta电位分析仪测定矿物表面的动电位。将矿样用玛瑙研钵研磨至粒径小于2 μm,每次称取20 mg矿样加入装有50 mL的超纯水的100 mL烧杯中,用磁力搅拌器搅拌3 min,再用HCl或NaOH调节pH,测定矿浆pH,最后加入一定浓度的捕收剂,搅拌4 min,使矿浆充分分散,沉降10 min之后取上层稀释的矿浆注入Coulter Delsa 440sx Zeta 电位分析仪的矩形电泳池内进行电位测定。每个样品测量 3 次,取其平均值。
1.3 吸附等温线
用紫外分光光度计(UV-3100)测定吡啶在波长为259 nm处的吸光度,从而计算出吡啶在矿物表面的吸附量。称取0.5 g矿样与40 mL蒸馏水在容量瓶混合中,加入所需浓度药剂并搅拌2 h,然后在4 000 r/min的转速下离心分离20 min。 过滤,测量上清液中吡啶的吸光度,通过标准曲线得到上清液吡啶浓度,进而计算出吡啶在矿物表面的吸附量。
1.4 表面微极性测试
将芘溶解在热水中至饱和,然后冷却到25 ℃过滤,制备芘原液。将油酸钠钠、芘原液和矿物混合成为矿浆,其中芘的浓度为6.84×10-7 mol/L。静置2 h使其达到悬浮平衡后用日立F-4500荧光分光光度计在特征波长335 nm时测定悬浮液的稳态发射光谱。
1.5 单矿物的浮选
单矿物浮选试验在XFG挂槽浮选机上进行,主轴转速为1 650 r/min。每次称取2.0 g单矿物放入40 mL浮选槽中,加35 mL蒸馏水,调浆1 min,再用HCl或NaOH调节pH至所需条件2 min后,加入捕收剂CPC,搅拌3 min,浮选4 min。泡沫产品和槽内产品分别烘干称质量并计算回收率。
2 试验结果与讨论
2.1 Zeta电位测试
在水溶液中,氧化钼表面等电点为1.9,因此,在大部分pH范围内其表面荷负电(见图1)。随着pH的增加其表面电位有下降趋势。而在溶液中加入7.0×10-4 mol/L吡啶捕收剂后,其表面电位变为正值,这是由于吡啶阳离子在荷负电的氧化钼表面的吸附:首先依靠静电作用力,吡啶阳离子与矿物表面结合使得矿物颗粒表面电位变大直至呈现电中性,而后通过疏水烃基之间的疏水化作用最终使得矿物表面荷正电。
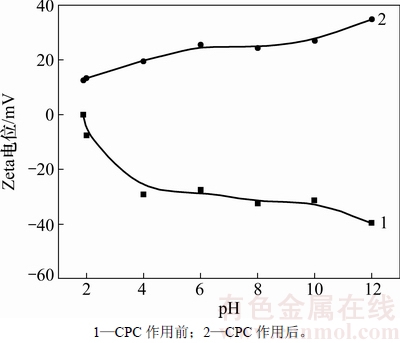
图1 氧化钼与CPC作用前后的表面动电位
Fig. 1 Zeta potentials of molybdite before and after treatment with CPC
2.2 吸附等温线
图2所示为吡啶在氧化钼表面的吸附等温线测试结果,据此可以获得不同平衡浓度下的吸附层结构信息。由图2可以看出:吸附等温线属于双平台型(Langmuir-sigmoid型),这说明矿物/捕收剂之间的吸附作用很强[12];氯化十六烷基吡啶的临界胶束浓度(CMC)为9.6×10-4 mol/L,当平衡浓度达到该临界值时,吸附达到饱和。吸附等温线可以简单划分为4个区域[13]:当捕收剂浓度较低的时候,捕收剂碳链间疏水作用较少可以忽略,主要是靠静电引力作用吸附到荷负电的矿物表面(A阶段)直至形成饱和的单层吸附,第1个吸附等温线平台开始出现(B阶段),此时的浓度称之为临界半胶束浓度(CHC,约6.4×10-4 mol/L);吸附量的第2次快速增加(C阶段)和第2个吸附等温线平台(D阶段)的出现是由于捕收剂的碳链相互作用出现双层吸附并趋于饱和。
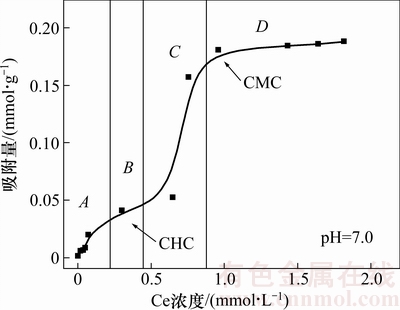
图2 吡啶在氧化钼表面的吸附等温线
Fig. 2 Adsorption isotherm of CPC on minerals
2.3 表面微极性研究
芘荧光探针是一种有效的获取矿物/水界面极性信息的手段[14-16],其发射光谱有5个特征峰,其中第1个和第3个特征峰值的比值I1/I3与探针所处微环境的极性密切相关。为了进一步研究吡啶捕收剂在氧化钼表面的吸附过程及吸附层结构,采用荧光探针测试对与捕收剂作用后的矿物表面进行微极性研究,结果见图3。由图3可知:特征参数I1/I3随着捕收剂浓度的增加而增加,初始值1.82对应芘在水中的I1/I3,而最小值接近1则是对应于芘在捕收剂胶束中(非极性环境)的I1/I3[17]。由图3可以看出:微极性测试曲线也可大致分为4个区域,平台出现处对应的捕收剂浓度也十分接近等温线分析中得到的CHC和CMC,进一步验证了吸附等温线的结果。

图3 吸附捕收剂的矿物表面微极性
Fig. 3 Microenvironment polarity of mineral absorbed collector
捕收剂在矿物表面吸附的4个阶段示意图如图4所示。在A阶段,吡啶阳离子通过静电作用自由排列在荷负点的氧化钼表面,与此同时溶液中的吡啶阳离子实际上也是自由分散的;随着捕收剂浓度的增加,越来越多的吡啶阳离子吸附在矿物表面的活性质点上,由于空间变小捕收剂的排列变得规则起来并最终形成亲固头基吸附在矿物表面而疏水尾端朝向溶液中的单层吸附(B阶段),此时疏水尾端之间的疏水化作用尚未发生,矿物表面疏水性最强;当捕收剂浓度进一步增加,疏水尾端之间的作用开始出现,致使半胶束吸附的发生(C阶段),这也是此阶段吸附量迅速增加的原因;在D阶段最终形成了双层吸附[18-19]。
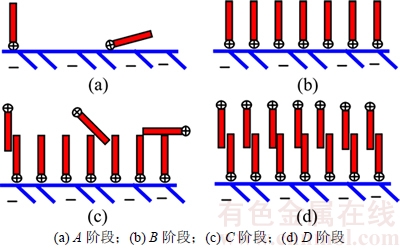
图4 捕收剂在矿物表面吸附的4个阶段示意图
Fig. 4 Schematic representation of collector on mineral surface at four steps
2.4 矿物可浮性试验
图5所示为pH、捕收剂用量与矿物浮选回收率的关系。在整个试验pH范围内,氧化钼的回收率均较高(>70%),表明吡啶捕收剂(0.4 mmol/L)对氧化钼具有较好的捕收效果。在中性pH条件下进行的捕收剂浓度对矿物浮选行为影响的实验表明,矿物回收率随吡啶捕收剂用量的增加呈现先增加后减小的趋势,在捕收剂浓度约为0.6 mmol/L时达到最大值,这对应于捕收剂单层饱和吸附的浓度(图2)。随后由于逐渐形成双层吸附,亲水头基朝向溶液使得矿物表面疏水性呈下降趋势,最终导致其浮选回收率的下降。由此可见,捕收剂在矿物表面的吸附层结构对其浮选行为具有重要影响。在利用离子型表面活性剂作为矿物捕收剂时应当严格控制其用量,否则可能起到反作用。
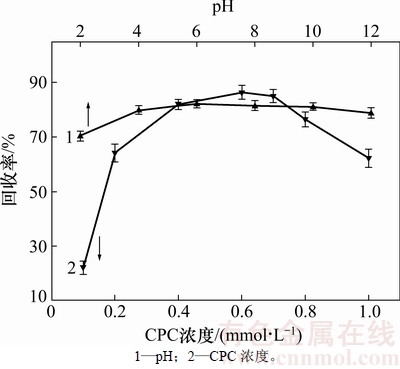
图5 pH和捕收剂浓度对氧化钼回收率的影响
Fig. 5 Flotation recoveries of molybdite as a function of pH and collector dosage
3 结论
1) 在吡啶捕收剂浓度较低时,捕收剂阳离子主要靠静电作用吸附作用在荷负电的氧化钼表面,而当浓度超过临界半胶束浓度时,疏水碳链之间的疏水化缔合起主要作用。
2) 吡啶捕收剂浓度不同,其在氧化钼表面的吸附层结构也不同,符合“四区域模型”,其中在B区由于矿物表面形成单层捕收剂分子吸附,表面疏水性最强。
3) 根据“四区域模型”,在浮选中离子型捕收剂添加量应该严格控制,当捕收剂浓度在临界半胶束浓度时获得最佳的浮选回收率。
参考文献:
[1] YAR-MUKHAMEDOVA G, VED M, SAKHNENKO N, et al. Iron binary and ternary coatings with molybdenum and tungsten[J]. Applied Surface Science, 2016, 383: 346-352.
[2] 程宝成, 李永峰, 谢克家, 等. 河南省钼矿资源特征、开发现状及产业发展对策[J]. 资源与产业, 2014, 16(1): 66-70.
CHENG Baocheng, LI Yongfeng, XIE Kejia, et al. Molybdenum resource features,development situation and industrial approaches in henan province[J]. Resources & Industries, 2014, 16(1): 66-70.
[3] WANG Zhen, XU Longhua, LIU Runqing, et al. Comparative studies of flotation and adsorption with cetyl pyridinium chloride on molybdite and fluorapatite[J]. International Journal of Mineral Processing, 2015, 143: 112-116.
[4] 王振, 孙伟, 徐龙华, 等. CPC在氧化钼表面吸附行为及分子动力学模拟[J]. 中南大学学报(自然科学版), 2013, 44(8): 3102-3107.
WANG Zhen, SUN Wei, XU Longhua, et al. Adsorption behavior of CPC on molybdite surface and molecular dynamics simulation[J]. Journal of Central South University (Science and Technology), 2013, 44(8): 3102-3107.
[5] LIU Weiping, XU Hui, YANG Xiyun, et al. Extraction of molybdenum from low-grade Ni-Mo ore in sodium hypochlorite solution under mechanical activation[J]. Minerals Engineering, 2011, 24(14): 1580-1585.
[6] AYLMORE M G. Treatment of a refractory gold-copper sulfide concentrate by copper ammoniacal thiosulfate leaching[J]. Minerals Engineering, 2001, 14(6): 615-637.
[7] GIBSON C E, HANSULD R, KELEBEK S, et al. Behaviour of ilmenite as a gangue mineral in the benzohydroxamic flotation of a complex pyrochlore-bearing ore[J]. Minerals Engineering, 2017, 109: 98-108.
[8] MARION C, JORDENS A, MCCARTHY S, et al. An investigation into the flotation of muscovite with an amine collector and calcium lignin sulfonate depressant[J]. Separation & Purification Technology, 2015, 149(10): 216-227.
[9] 孙伟, 王振, 曹学锋, 等. 某镍钼矿浮选试验研究[J]. 金属矿山, 2012, 41(1): 97-99.
SUN Wei, WANG Zhen, CAO Xuefeng, et al. Experiment study on flotation of nickele-molybdenum ore[J]. Metal Mine, 2012, 41(1): 97-99.
[10] SOLFERINO G, ANDERSON A J. Thermal reduction of molybdite and hematite in water and hydrogen peroxide-bearing solutions: Insights on redox conditions in Hydrothermal Diamond Anvil Cell (HDAC) experiments[J]. Chemical Geology, 2012, 322/323: 215-222.
[11] MEHDILO A, IRANNAJAD M. Evaluation of pyrolusite flotation behavior using a cationic collector[J]. Journal of Mining Science, 2014, 50(5): 982-993.
[12] GILES C H, MACEWAN T H, NAKHWA S N, et al. Studies in adsorption, Part XI: a system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids[J]. Journal of the Chemical Society (Resumed), 1960, 786: 3973-3993.
[13] LI Feng, YE Lanlan, LI Yujiang, et al. Investigation into the adsorption of partially hydrolyzed polyacrylamide onto formed magnesium hydroxide particles[J]. Rsc Advances, 2016, 6(37): 31092-31100.
[14] NOSAKA Y, NISHIKAWA M, NOSAKA A Y. Spectroscopic investigation of the mechanism of photocatalysis[J]. Molecules, 2014, 19(11): 18248-18267.
[15] OTTAVIANI M F, VENTURI F, POKHREL M R. Physicochemical studies on the adsorption properties of asbestos: 2. an EPR and fluorescence study on the adsorption of pyrene[J]. Journal of Colloid & Interface Science, 2001, 238(2): 371-380.
[16]  L, NOVO M, AL-SOUFI W. Fluorescence emission of pyrene in surfactant solutions[J]. Advances in Colloid and Interface Science, 2015, 215: 1-12.
L, NOVO M, AL-SOUFI W. Fluorescence emission of pyrene in surfactant solutions[J]. Advances in Colloid and Interface Science, 2015, 215: 1-12.
[17] PISTOLIS G, MALLIARIS A, TSIOURVAS D, et al. Poly (propyleneimine) dendrimers as pH-sensitive controlled-release systems[J]. Chemistry-a European Journal, 1999, 5(5): 1440-1444.
[18] WANG Zhen, XU Longhua, WANG Jinming, et al. A comparison study of adsorption of benzohydroxamic acid and amyl xanthate on smithsonite with dodecylamine as co-collector[J]. Applied Surface Science, 2017, 426: 1141-1147.
[19] DAN A, GOCHEV G, MILLER R. Tensiometry and dilational rheology of mixed β-lactoglobulin/ionic surfactant adsorption layers at water/air and water/hexane interfaces[J]. Journal of Colloid & Interface Science, 2015, 449: 383-391.
(编辑 赵俊)
收稿日期:2016-12-14;修回日期:2017-04-12
基金项目(Foundation item):国家自然科学基金资助项目(51504199,51304162)(Projects(51504199, 51304162) supported by the National Natural Science Foundation of China)
通信作者:王振,讲师,从事有色金属及非金属选矿等研究;E-mail:wangzhen@swust.edu.cn