
J. Cent. South Univ. (2018) 25: 2962-2970
DOI: https://doi.org/10.1007/s11771-018-3966-6

Spray deposition of FeCrNiMn and high carbon steel coatings by thermite reaction
CHEN Gang(陈刚)1, SHEN Shu-cheng(沈书成)1, NI Song(倪頌)2, ZHOU Chen-shang(周承商)2
1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: A novel surface cladding technique was developed to prepare the FeCrNiMn alloy and high carbon steel cladding layers, and the microhardness, bonding strength, abrasion wear and corrosion resistance were investigated. The microstructures of the cladding layers were analyzed by using X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive spectrometry (EDS). The results show that the bonding strength between the substrate and the two cladding layers were (432.6±21) and (438.3±12)MPa,respectively. Vickers hardness values of the two cladding layers were HV 418.5 and HV 329.6, respectively. The corrosion current densities of the two coatings were 2.926×10–6 and 6.858×10–6A/cm2 after electrochemical corrosion test in 3.5% NaCl solution, and the wear rate were 1.78×10–7 and 1.46×10–6mm3/mN after sliding wear test, respectively. This indicates that a well metallurgical bonding between the coating and the substrate was achieved, the abrasion wear and corrosion resistance of both coatings had been greatly improved compared with the substrate. The novel cladding technology is promising for preparing wear-and-corrosion resistant coatings.
Key words: spray deposition; thermite reaction; coating; corrosion property; wear property
Cite this article as: CHEN Gang, SHEN Shu-cheng, NI song, ZHOU Chen-shang. Spray deposition of FeCrNiMn and high carbon steel coatings by thermite reaction [J]. Journal of Central South University, 2018, 25(12): 2962–2970. DOI: https://doi.org/10.1007/s11771-018-3966-6.
1 Introduction
Superior surface properties of materials such as high anti-wear property and high corrosion resistance are required for engineering applications. Laser cladding [1–4], electron beam cladding [5], thermal spraying [6] and some other surface treatment techniques [7, 8] have been widely applied to achieve good surface properties for materials. For example, laser cladding has been used to prepare Ni-based and Co-based alloy coatings [9, 10], cemented carbide coatings [11], intermetallic compounds coatings [12, 13] and high-entropy alloy coatings [14], significantly improving the modification of components like mold, turbine blades, turbine rotor and drill pipe [15–18]. Thermal spraying has been used to fabricate the coatings for most engineering materials including a variety of metals and alloys, ceramics, plastics and non-metallic materials [19–22]. However, the narrow single-pass of laser cladding [23] makes it difficult for the cladding layer to connect with each other, which limits the preparation of large-area cladding. Because of the temperature gradient across the section, defects [24] appeared easily at the boundary between the cladding layers and the substrate during rapid heating and subsequent rapid cooling [25].
Therefore, it is important to develop a new technique for producing large-area and thicker coating at high efficiency and quality so as to improve the abrasion resistance and corrosion resistance of large-sized structural components.
We developed a novel surface cladding coating technique, in which high temperature alloy melt obtained by thermite reaction is used to form spray droplets under inert gas atomization, and then sprays directly onto the substrate to form a cladding layer. Because the temperature of melt during thermite reaction can reach above 2500°C, the as-atomized droplet still has the ability to remelt the substrate surface when it impacts and sputters onto the substrate, so the cladding coating has good metallurgical bonding with the substrate. Meanwhile, the coatings in fact prepared by spray deposition also have rapid solidification structure, and thus, excellent mechanical properties [26, 27]. In the present work, FeCrNiMn alloy coatings on the 45# steel substrate were prepared by this technique.
2 Experimental
2.1 Raw materials and preparation of coatings
Figure 1 presents the schematic view of the spray deposition device for preparation of coating. The mixed powders of thermite reaction agent in the crucible were ignited by the induction heating device to form high temperature alloy melt. The by-product of the thermite reaction Al2O3 as melt slag floated on the melt. After the temperature of the melt reaching about 2000°C, the stopper was unplugged to let the melt enter into the atomizer through the melt delivery tube, and was atomized into fine droplets by the high pressure gas. The droplets deposited on the substrate pre-heated at 400°C to form cladding coating by scanning deposition processing.
The commercially available Fe2O3 powder, Al powder, graphite powder and alloy element powders were used as thermite reaction agent to prepare high carbon steel coating with the designed composition of 0.7% C, 1.00% Mn and 0.35% Si (mass fraction). For preparation of alloy steel coating, 5.00% Cr powder and 5.00% Ni powder were added in the thermite reaction.
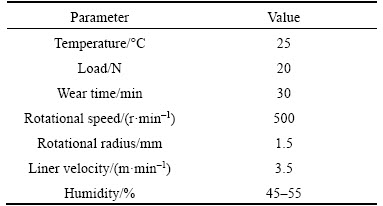
Before spray deposition, the substrate was ground using coarse sand paper to remove the oxide layer on the surface and to make the surface rough. The substrate was pre-heated up to about 400°C by a flat plate high-frequency induction heater before spray deposition. Table 1 exhibits the parameters of spray deposition cladding.

Figure 1 Schematic illustration of experimental setup (1–Stopper; 2–Exhaustsystem; 3–Protection cover; 4–Induction heating furnace; 5–Crucible; 6–Melt delivery tube; 7–Atomizer; 8–Spray flow; 9–Cladding coating; 10–Matrix; 11–Scanning direction; 12–Surface induction heater; 13–Baffle of surface induction heater; 14–High pressure atomizing gas; 15–Superalloy melt; 16–Slag of alumina; 17–Infrared thermometer window; 18–Infrared radiation pyrometer)
Table 1 Parameters of spray deposition cladding

2.2 Wear and corrosion tests
A ball-on-disk tribometer (SFT-2M, China) was used for friction and wear tests. The commercial AISI52100 ball(HRC 62-65, diameter of 3mm)as the upper specimens, substrate and coatings were served as the lower specimen. Before the tests, the samples were ground with emery paper No. 600, No. 1200 and No. 2000 and then ultrasonically cleaned in ethanol for about 10 min. Table 2 shows the parameters of friction and wear tests. The friction coefficient curves of the specimens were recorded by a computer connected with the tribometer. The 3D topography and cross-sectional area were obtained by using Wfko NT9100 surface profiler. The wear rates (W) of the specimen were given as W=V/LF1, where V is the wear volume loss in mm3, L is the total sliding distance in m, and F1 is the applied load in N.
Table 2 Friction and wear tests parameters
Tafel polarization curves of the samples were acquired by using a CHI660C electrochemical workstation in 3.5 wt% NaCl solution. A three-electrode system was used during corrosion resistance tests. The specimens with an area of 0.5cm2 acted as the working electrode, a platinum plate posed as the counter electrode and the reference electrode was a saturated calomel electrode (SCE). Before electrochemical tests, the samples were immersed in the medium for 30min. The Tafel curves were obtained by computer contacted to the electrochemical workstation at a scan rate of 1mV/s.
2.3 Characterization of coating
The morphologies of the feed stock powders, the spray deposition coatings, the fracture surface and wear scars were analyzed by scanning electron microscope (SEM, Quanta-200 and JSM-6700F). X-ray diffraction (XRD, Philips X’ Pert-MPD) was used to detect the phase compositions of the two cladding layers with Cu-Kα radiation. The composition of the coating was measured by energy dispersive spectroscopy (EDS) and inductively coupled plasma (ICP). Vickers hardness of the spray deposited coatings was measured by MH-5-VM hardness tester under a load of 9.8N and duration time of 15s. Each hardness value was averaged from 5 individual measurements.
2.4 Bonding strength of coatings
The bonding strength between the coatings and the substrate was measured for four times using top breaking test. The top breaking test was adopted to measure the shear force load on the joint surface between the substrate and the coating. The bonding strength of the specimen with dimensions of 2.2mm×2.2mm×20mm was calculated as P=F2/S, where F is the applied load and S is the cross-sectional area. The schematic illustration of the top breaking test is shown in Figure 2. The top breaking test machine was manufactured by Hunan Bichamp Cutting Technology Co., Ltd. To detect the weld strength of the tooth and the back of the bimetal band saw blades, the specimens were fixed on a fixture with a man drill to exert a shear force to cut the coating and the substrate, and the shear force was recorded through the sensor device. The maximum stress during the test is considered the shear breaking strength.
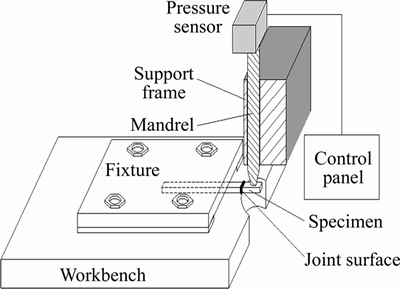
Figure 2 Schematic illustration of top breaking test
3 Results and discussions
3.1 Interfacial bonding state of coatings
Figure 3 shows the SEM images of the cross section in the as-spray deposited coatings and details of the interface morphology between the coatings and substrate. As shown in Figures 3(a) and (b), coatings in a thickness of about 6 mm with highly dense microstructures were obtained. As shown in Figures 3(c) and (d), no pores or cracks were found on the surface of the coatings. The bonding strength between the substrate and the coatings was measured by the aforementioned top breaking test. The average bonding strengths for the two coatings are (432.6±21)MPa and (438.3±12)MPa, respectively. Figure 4(a) shows the EDX line scanning results of the interface between the carbon steel coating and the substrate. As can be seen from the image, the carbon content slowly decreases from the coating to the substrate. Figure 4(b) shows the line scanning results of the bonding surface between the alloyed steel coating and the substrate. It can be observed that the contents of element C, Fe and Mn remain basically unchanged, but the contents of Cr and Ni decrease gradually from the coating to the substrate, indicating that elemental diffusion occurred on the interface. The above- mentioned phenomenon clearly demonstrates that a strong metallurgical bonding between the coating and the substrate is achieved.
Figure 5 shows the SEM images of the fractured surfaces of the carbon steel coating (Figures 5(a) and (c)) and alloy steel coating (Figures 5(b) and (d)). It can be seen that a large number of cleavage steps and river patterns form on the fracture surfaces of the carbon steel coating. Many of the cleavage cracks propagate from one grain into neighboring grains, as marked with black arrows. Some parallel cleavage steps are marked with white arrows in Figure 5(c). It shows that cleavage fracture occurred in the carbon steel coating. This can be contributed to the thermal effects leading to obvious grain coarsening in the substrate and the higher carbon content near the joint surface. The fracture mode of the alloy steel coating is determined to be quasi-cleavage fracture due to the formation of a lot of dimples marked with black arrows and a bit of parallel cleavage steps marked with white arrows are observed on the fracture surface along the shearing direction in Figure 5(d).

Figure 3 Boundary zone morphology of coatings:
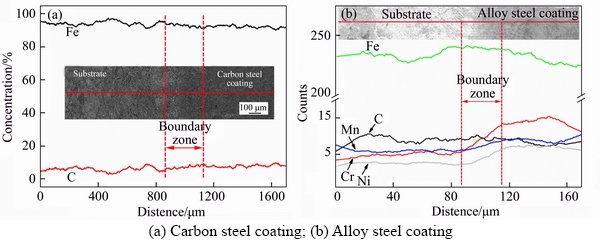
Figure 4 EDS line scans results of cross section of boundary zones:

Figure 5 SEM images of fracture surfaces of coatings:
3.2 Microstructure and properties of coatings
Figures 6(a) and (b) show the SEM images of the carbon steel and alloy steel coatings, respectively. It is clear that pearlitic structure formed in the carbon steel coating, as shown in Figure 6(a), and the average slice thickness is approximately 0.35μm. The grains size of carbon steel coating is around 10–20μm. As shown in Figure 6(b), some upper bainitic structure was observed with the addition of Cr and Ni, and the grain size of alloy steel coating is around 5–15μm. Both the pearlitic and upper bainitic are typically structures formed during rapid solidification in which the diffusion of carbon is inhibited due to the high solidification rate. The EDS and ICP analysis results of the coatings are presented in Table 3. The mass fractions of Mn and Si elements in the carbon steel coating are 0.95% and 0.30%, respectively. The mass fractions of Cr, Ni and Mn elements in the alloy steel coating are 2.80%, 3.89% and 1.28%, respectively. Figure 7 shows the XRD results of the two cladding layers. Fe-based solid solution (Fe–Cr–Ni, Fe–Mn), carbide and a small number of oxides are detected in the alloy steel coating. Fe-based solid solution (Fe–Mn), carbide (MxCy) and a small number of oxides are observed in the carbon steel coating.
Figure 8 shows the Vickers hardness of the substrate and the as-spray deposited coatings. The hardness of the substrate is measured to be HV 243.1, while the hardness of the carbon steel coating is improved to HV 329.6, and the hardness of the alloy steel coating is further increased to HV 418.5. The existence of a small amount of oxide in the coating, solid solution strengthening and rapid solidification effect contributes to the high hardness [28]. As for the carbon steel coating, higher hardness than the matrix is mainly due to the rapid solidification effect [29]. The higher hardness of the alloy steel coating than the carbon steel coating can be attributed to the solution strengthening effect of the Cr and Ni elements.

Figure 6 SEM morphologies of coatings:
Table 3 EDS result of as-sprayed deposition coatings (mass fraction, %)

Figure 7 XRD patterns of carbon steel coating and alloy steel coatings

Figure 8 Microhardness of substrate and spray deposited coatings
3.3 Wear and corrosion performance
Figure 9(a) shows the curves of friction coefficients of the substrate steel and the spray deposited coatings sliding against AISI52100 steel ball. It can be found that the friction coefficient of the substrate is around 0.48. Carbon steel coating and alloy steel coating exhibit high coefficients up to 0.55 and 0.60, respectively. Comparing with the friction coefficient of the substrate which keeps almost constant, that of the carbon steel coating firstly drops to a low level and then raises to a constant value. But, the alloy steel coating exhibits a gradual upward trend. Figure 9(b) gives out the wear rates of the spray deposited coatings and substrate. The substrate exhibits a very high wear rate of 5.02×10–6mm3/mN. But the spray deposited alloy steel coating exhibits an abrupt decrease up to 1.87×10–7mm3/mN, which is much lower than that of the carbon steel coating, 1.46×10–6mm3/mN. The above results show that the wear rate of the coating decreases with the increase of hardness,which is similar to the related reports [30].

Figure 9 Curves of friction coefficients (a) and wear rates (b) of substrate and spray deposition coatings
The worn surfaces of the spray deposited coatings and the substrate were further characterized to understand the wear mechanisms. Figure 10 shows the SEM images of the worn surfaces of substrate, spray deposited carbon steel coating, and alloy steel coating. In Figure 10(a), the substrate exhibits debris and deep groove when sliding against AISI52100, indicating a serious adhesion wear. In Figure 10(b), the carbon steel coating exhibits a small amount of metal fall-offs and little parallel grooves. In Figure 10(c), a small number of shallow groove and dark area is observed and no metal fall-offs and adhesive pits are found, which indicates that with the addition of Cr and Ni elements, the alloy steel coating exhibits excellent abrasion resistance. The black area may be due to the shedding of a small amount of oxide remaining in the coating.
Figure 11 shows the Tafel polarization curves of the specimens in 3.5 wt.% NaCl solution. The current densities of the carbon steel coating and alloy steel coating are lower than those of the substrate in the polarization curves. The corrosion potential and corrosion current density changes from 4.361×10–5A/cm2 and –0.588V (vs SCE) of the substrate to 6.858×10–6A/cm2 and –0.718V (vs SCE) of the spray depositing carbon steel coating, which demonstrates that the coatings produced by spay deposition cladding can provide good protection for substrates. Furthermore, the corrosion resistance of the alloy steel coating is significantly increased with the addition of Cr and Ni elements, the corrosion current density and corrosion potential are further decreased to 2.926×10–6A/cm2 and –0.377V (vs SCE).

Figure 10 SEM images of worn surfaces:
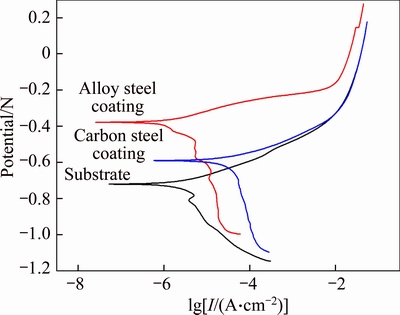
Figure 11 Polarization curves of substrate and spray deposited coatings
4 Conclusions
1) Spray deposition combining with thermite reaction is successfully used to prepare high quality coatings at high efficiency.
2) The bonding strengths for the alloy steel coating and carbon steel coating are (432.6±21)MPa and (438.3±12)MPa, respectively. A well metallurgical bonding between the coating and the substrate is achieved.
3) The average hardness values of the alloy steel coating, carbon steel coating and substrate are HV 418.5, 329.6, and 243.1, the corrosion current density of them are 2.926×10–6, 6.858×10–6, 4.361×10–5A/cm2 after electrochemical corrosion test in 3.5% NaCl solution, the wear rates of them are 1.78×10–7, 1.46×10–6 and 5.02×10–6 mm3/mN, respectively. The hardness, electrochemical corrosion resistance and wear resistance of the alloy steel coating and carbon steel coating clearly increase compared with those of the substrate.
References
[1] MANNA I, MAJUMDAR J D, CHANDRA B R, NAYAK S, DAHOTRE N B. Laser surface cladding of Fe-B-C, Fe-B-Si and Fe-BC-Si-Al-C on plain carbon steel[J]. Surface & Coatings Technology, 2006, 201(1, 2): 434–440.
[2] ZHOU Sheng-feng, HUANG Yong-jun, ZENG Xiao-yan, HU Qian-wu. Microstructure characteristics of Ni-based WC composite coatings by laser induction hybrid rapid cladding [J]. Materials Science and Engineering A, 2008, 480(1, 2): 564–572.
[3] WANG Shan-li, CHENG Jing-chang, YI Cheng-xun, KE Li-ming. Corrosion resistance of Fe-based amorphous metallic matrix coating fabricated by HVOF thermal spraying [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(1): 146–151.
[4] LIU Yong, LIU Su-qin, WANG Shun-xing. Microstructure and abrasion wear behavior of Ni-based laser cladding alloy layer at high temperature [J]. Journal of Central South University of Technology, 2005, 12(4): 403–405.
[5] ABE N, MORIMOTO J, TOMIE M, DOI C. Formation of WC-Co layers by an electron beam cladding method and evaluation of the layer properties [J]. Vacuum, 2000, 59(1): 373–380.
[6] KULU P, ZIMAKOV S. Wear resistance of thermal sprayed coatings on the base of recycled hardmetal [J]. Surface & Coatings Technology, 2000, 130(1): 46–51.
[7] ISHIZAKI T, HIEDA J, SAITO N, TAKAI O. Corrosion resistance and chemical stability of super-hydrophobic film deposited on magnesium alloy AZ31 by microwave plasma-enhanced chemical vapor deposition [J]. Electrochimica Acta, 2010, 55(23): 7094–7101.
[8] EZHILSELVI V, NITHIN J, BALARAJU J N, SUBRAMANIAN S. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy [J]. Surface & Coatings Technology, 2016, 288: 221–229.
[9] GUO Huo-ming, WANG Qian, WANG Wen-jian, GUO Jun, LIU Qi-yue, ZHU Min-hao. Investigation on wear and damage performance of laser cladding Co-based alloy on single wheel or rail material [J]. Wear, 2015, 328: 329–337.
[10] NIE P, OJO O A, LI Z. Modeling analysis of laser cladding of a nickel-based superalloy [J]. Surface & Coatings Technology, 2014, 258: 1048–1059.
[11] PAUL C P, KHAJEPOUR A. Automated laser fabrication of cemented carbide components [J]. Optics & Laser Technology, 2008, 40(5): 735–741.
[12] YU You-jun, ZHOU Jian-song, REN Shu-fang, WANG Ling-qian, XIN Ben-bin, CAO Si-long. Tribological properties of laser cladding NiAl intermetallic compound coatings at elevated temperatures [J]. Tribology International, 2016, 104: 321–327.
[13] LIU Kun, LI Ya-jiang, WANG Juan. In-situ reactive fabrication and effect of phosphorus on microstructure evolution of Ni/Ni–Al intermetallic composite coating by laser cladding [J]. Materials & Design, 2016, 105: 171–178.
[14] CHENG Jiang-bo, LIU Dan, LIANG Xiu-bing, CHEN Yong-xiong. Evolution of microstructure and mechanical properties of in situ synthesized TiC–TiB2/CoCrCuFeNi high entropy alloy coatings [J]. Surface & Coatings Technology, 2015, 281(7): 109–116.
[15] YAN Hua, ZHANG Jie, ZHANG Pei-lei, YU Zhi-shui, LI Chong-gui, XU Pei-quan, LU Yun-long. Laser cladding of Co-based alloy/TiC/CaF2, self-lubricating composite coatings on copper for continuous casting mold [J]. Surface & Coatings Technology, 2013, 232(1): 362–369.
[16] SHEPELEVA L, MEDRES B, KAPLAN W D, BAMBERGER M, WEISHEIT A. Laser cladding of turbine blades [J]. Surface & Coatings Technology, 2000, 125(1–3): 45–48.
[17] WENG Fei, CHEN Chuan-zhong, YU Hui-jun. Research status of laser cladding on titanium and its alloys: A review [J]. Materials & Design, 2014, 58(6): 412–425.
[18] QUAZI M M, FAZAL M A, HASEEB A S M A, YUSOF FMASJUKI H H, ARSLAN A. Effect of rare earth elements and their oxides on tribo-mechanical performance of laser claddings: A review [J]. Journal of Rare Earths, 2016, 34(6): 549–564.
[19] SHAKHOVA I, MIRONOV E, AZARMI F, ASFONOV A. Thermo-electrical properties of the alumina coatings deposited by different thermal spraying technologies [J]. Ceramics International, 2017, 43(17): 15392–15401.
[20] AKIN S R K, TURAN S, GENCOGLU P D, MANDAL H. Effect of SiC addition on the thermal diffusivity of SiAlON ceramics [J]. Ceramics International, 2017, 43(16): 13469– 13474.
[21] DAS P, PAUL S, BANDYOPADHYAY P P. Preparation of diamond reinforced metal powders as thermal spray feedstock using ball milling [J]. Surface & Coatings Technology, 2016, 286: 165–171.
[22] KIRBIYIK F, GOK M G, GOLLER G, FIRBIYIK F, GOK M G, GOLLER G. Microstructural, mechanical and thermal properties of Al2O3/CYSZ functionally graded thermal barrier coatings [J]. Surface & Coatings Technology, 2017, 329.
[23] LAI Quan, ABRAHAMS R, YAN Wen-yi, SOODI M. Investigation of a novel functionally graded material for the repair of premium hypereutectoid rails using laser cladding technology [J]. Composites Part B Engineering, 2017, 130: 174–191.
[24] ERFANMANESH M, ABDOLLAHPOUR H, MOHAMMADIANSEMNANI H, SHOJARAZAVI R. An empirical-statistical model for laser cladding of WC-12Co powder on AISI 321 stainless steel [J]. Optics & Laser Technology, 2017, 97: 180–186.
[25] HSU W L, YANG Y C, CHEN C Y, YEH J W. Thermal sprayed high-entropy NiCo0.6Fe0.2Cr1.5SiAlTi0.2 coating with improved mechanical properties and oxidation resistance [J]. Intermetallics, 2017, 89: 105–110.
[26] TENG Jie, LI Hua-pei, CHEN Gang. Wear mechanism for spray deposited Al-Si/SiCp composites under dry sliding condition [J]. Journal of Central South University, 2015, 22(8): 2875–2882.
[27] ZHAO Guo-ji, WEN Guang-hua, SHENG Guang-min, JING Yan-xia. Effects of rapid solidification process and 0.1%Pr/Nd addition on characteristics of Sn-9Zn solder alloy and interfacial properties of Cu/solder/Cu joints [J]. Journal of Central South University, 2016, 23(8): 1831–1838.
[28] BADISCH E, KATSICH C, WINKELMANN H, FRANEK F, ROY M. Wear behaviour of hardfaced Fe-Cr-C alloy and austenitic steel under 2-body and 3-body conditions at elevated temperature [J]. Tribology International, 2010, 43(7): 1234–1244.
[29] MANAF A, LEONOWICZ M, DAVIES H A, BUCKLEY R A. Effect of grain size and microstructure on magnetic properties of rapidly solidified Fe82.4Nd13.1B4.5 alloy [J]. Journal of Applied Physics, 1991, 70(10): 6366–6368.
[30] LUO Xi-xi, YAO Zheng-jun, ZHANG Ping-ze, CHEN Yu, YANG Hong-qin, WU Xiao-feng, ZHANG Ze-lei, LIN Yu-hua, XU Shang-jun. Tribological behaviors of Fe-Al-Cr-Nb alloyed layer deposited on 45 steel via double glow plasma surface metallurgy technique [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 3694–3699.
(Edited by FANG Jing-hua)
中文导读
基于铝热反应的喷射熔覆FeCrMn合金与高碳钢涂层微观组织与性能
摘要:本文提出了一种新颖的表面熔覆涂层技术, 并通过此技术制备了FeCrMn合金与高碳钢涂层,并对比研究了涂层的显微硬度、结合强度、耐磨损和耐腐蚀性能。采用X射线衍射(XRD)、扫描电子显微镜(SEM)和能谱(EDS)对涂层的微观组织、结构及元素分布进行了分析。两种涂层与基体的结合强度分别达到了(432.6±21)和 (438.3±12) MPa,维氏硬度分别达到了HV 418.5和HV 329.6,两种涂层在3.5% NaCl溶液中的电化学腐蚀电流密度分别为2.926×10-6 和6.858×10-6 A/cm2,磨损速率分别为1.78×10–7和1.46×10–6 mm3/mN。研究结果表明:本技术制备的涂层能够与基体达到良好的冶金结合同时具有优异的耐腐蚀耐磨损性能,可望发展成为一种制备耐蚀耐磨涂层的新方法。
关键词:喷射沉积;铝热反应;涂层;腐蚀性能;磨损性能
Foundation item: Project(2016JJ2025) supported by the Natural Science Foundation of Hunan Province, China; Project(U1560105) supported by the National Natural Science Foundation of China
Received date: 2018-01-16; Accepted date: 2018-07-20
Corresponding author: CHEN Gang, PhD, Professor; Tel: +86-13873120655; E-mail: chengang@hnu.edu.cn; ORCID: 0000-0002-0186- 9766