
Grain-refining persistency of Al-based alloy produced by electrolyzing
LIU Zhi-yong(刘志勇), WANG Ming-xing(王明星), LIU Zhong-xia(刘忠侠),
WENG Yong-gang(翁永刚), SONG Tian-fu(宋天福), ZUO Xiu-rong(左秀容), YANG Sheng(杨 昇)
Key Laboratory of Materials Physics, Ministry of Education, Physics Department, Zhengzhou University,
Zhengzhou 450052, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The Al-based alloy with equiaxed grains was directly produced in the industrial aluminum electrolyzer. The varieties of grain features and grain sizes vs the re-melting times of this alloy were investigated. The grain features and mechanical properties of A356 alloy made from this alloy during the several re-melted times were also studied. The results show that the Al-based alloy after re-melted for 6 times, and A356 after re-melted for 3 times, both remained and refined grains and A356 alloy could achieve favorable mechanical properties. All these should be attributed to the electrolyzing procedure, in which nucleates, such as Al3Ti, are distributed fully and even in the based alloy. Producing Al-based alloy in the industrial electrolyzer provides a new, efficient and practical grain-refining route.
Key words: A356 alloy; grain-refining; electrolyzing; re-melting effect
1 Introduction
Master alloys of Al-Ti and B or C are widely used as grain refiners in aluminum alloys to improve the mechanical properties, feeding and surface finish, to reduce hot-tearing, and to distribute porosities evenly [1-2]. Instead of pre-fabricating the master alloys and their addition to aluminum alloy for grain refining, LIU et al[3] produced the self-grain-refined Al-based alloy through directly electrolyzing the titanium dioxide in cryolite-aluminum melts in the industrial electrolyzer. This new procedure was efficient in aluminum’s and its alloy’s grain-refining, and could also be put into industrial production[4]. Our recent researches[5-6] showed that this new route had many advantages over using the ordinary master alloy of Al-Ti in refining the aluminum, and could obtain the comparable grain refinement to the Al-Ti-B master alloy.
In industrial practice, considerable amount of aluminum products are usually be recalled and re-utilized for many times. Furthermore, the running and feeding parts of cast alloys—usually accounts for over 30% of the total ingots[7], must be re-melted. As the Al-based alloy directly produced in electrolyzer should be used as starting materials in fabrication other aluminum alloy, its grain-refinement effect vs melting time should be evaluated. The influence of melting times on the grain refinement of the alloys made from the starting material should also be studied. This work reports the grain refinements of the Al-based alloy and A356 alloy made from the based alloy verse the corresponding re-melting times. The varieties of mechanical properties of A356 alloy are also studied to further evaluate the grain- refining persistency of the application of Al-based alloy.
2 Experimental
The Al-based alloy used in this study was directly electrolyzed in the industrial electrolyzer, with the composition (mass fraction, %) of Ti 0.214, Cu 0.025, Mg 0.008, Fe 0.082, Si 0.040 and Al balance. The Al-base alloy, together with crystal silicon, magnesium ingots and the Al-7%Sr modifier were used to produce the A356 alloy, with the nominal compositions of Si 6.50-7.50, Mg 0.45-0.60, Ti 0.17-0.20, Fe 0-0.12, Sr 0.01-0.02, and Al balance.
The maximum melting temperature was 1 063 K. After degassed with argon gas at 1 013 K, the melts were poured into the central cavity (30 mm in diameter and 70 mm in depth) within the pre-heated cylindrical graphite mold with 70 mm in diameter and 90 mm in depth. These samples were for macro-and micro-analyses. The A356 melts were also poured into the permanent mould to obtain the standard tensile specimens for mechanical properties measurement. Cast A356 alloys were solution-treated at 810 K for 6 h, and then quenched into water at 330 K, followed by aging at 435 K for 8 h.
The samples used for macro-analysis were the bottom surfaces of ingots. After mechanically grounded, they were etched with the reagent (HCl?H2O? HNO3? FeCl3=200 mL?100 mL?180 mL?60 g). The samples for micro-analysis were the central sections of ingots. After mechanically grounded and polished, they were etched with the 0.5% HNO3 solution. The grain size was measured by the linear intercept technique[8]. The mechanical properties were conducted on MTS 810 Materials Testing System. JSM-6700F SEM was used to analyze the fracture features.
3 Results and discussion
It is found that titanium and silicon for Al-based alloy and A356 alloy remain in the almost same contents during the re-melting procedure, while Mg and Sr decrease by 20% after every re-melting of A356 alloy. So, the Al-based alloy was repeatedly melted for six times without adding any other elements. As to A356 alloy, about excess 20% Mg and Sr were added to the melts for every re-melting treatment in order to maintain the persistency of chemical compositions.
As shown in Fig.1, after re-melted below 1 063 K, even for more than 6 times, this Al-based alloy remains in fine equiaxial grains, which can be categorized as 1st degree of grains. The grain feature and distribution do not change greatly after re-melted for 6 times. As shown in Fig.2, it can also be found that after re-melted for 6 times, the grain sizes do not increase greatly, almost remaining the constant around 115 μm.
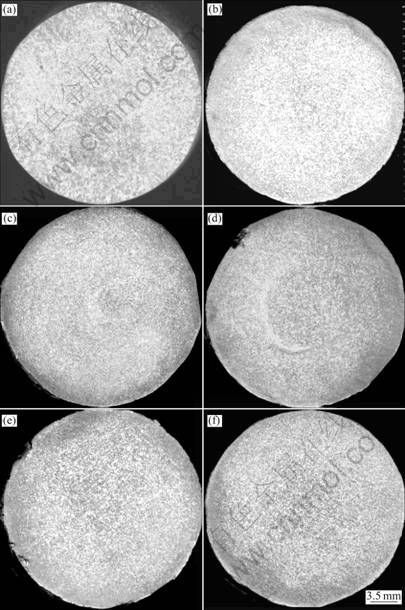
Fig.1 Marcographs of Al-based alloy (Ti: 0.214%) vs melting times: (a) 1st; (b) 2nd; (c) 3rd; (d) 4th; (e) 5th; (f) 6th

Fig.2 Curve of grain size vs melting time
Under the ordinary solidification, the electrolyzed ingots of pure aluminum from the cooling line reveal in coarse columnar grains. Due to little solute elements and absence of potent nuclei, the α(Al) dendrites can develop freely into large trunks and multi-order branches along the preferred crystallographic direction[8].
During the electrolyzing procedure of the Al-based alloy at 1 350 K, solute titanium atoms can be uniformly distributed in the aluminum melts under the force of intense electromagnetic field[3]. In the following solidification, solute titanium will be segregated at the advancing solid/liquid interface. This will lead to the formation of potent fine particles in the form of TiAl3 etc, and some of them may act as the nuclei of α(Al)[9]. So the equiaxial grains instead of columnar grains reveal in the Al-based alloy.
Sufficient nuclei of α(Al) could be distributed evenly in the directly electrolyzed Al-based alloy. During the re-melting procedure, the maximum melting temperature is just 1 063 K, so most of these nuclei can not be re-melted and enough nuclei would remain in the melts. In the following cooling procedure, the residual solute titanium can supply enough constitutional under-cooling to activate those remained nuclei to form sufficient α(Al) grains. The evenly distributed nuclei will induce the fine equiaxial grains. During the re-melting treatment, titanium content remains the constant, which will maintain the enough amount of nuclei. The further study[5-6] showed that the nucleant particles seldom aggregate at the bottom of the ingots. The un-changeable content of titanium and evenly distribution of titanium in the Al-based alloy ensure its refinement effect on the aluminium grains. All these should be attributed to the electrolysis procedure.
The above results yield that the Al-based alloy can be used for no less than 6 times, maintaining its favorite grain refining effect. But this just means that the grain-refining persistency can be sustained without the influence of other elements under the practical re-melted treatment. Cast A356 alloy is wildly used in automotive industry because of excellent cast-ability and mechanical properties[10-11]. In early research[4], the Al-based alloy showed better grain refinement response to silicon content below 12%. In order to evaluate the grain-refining persistency for the industrial application of Al-based alloy, A356 alloy is chosen as the target alloy.
As shown in Fig.3, it can be found that the A356 samples during re-melted for three times totally reveal in equiaxial grains, although the grain size increases a little with the melting times. Particles in types of TiAl3 or Ti(Al3-xSix) types may be acted as the nuclei for Al-Si alloy made from the based alloy[4]. As to A356 alloy, solute titanium plus silicon can supply the sufficient under- cooling to activate the potent nuclei. The titanium remaining the constant during the re-melting treatment, can maintain the corresponding amounts of TiAl3 or Ti(Al3-xSix) particles[4] in the melts. This ensures the grain-refining persistency for the application of the alloy.
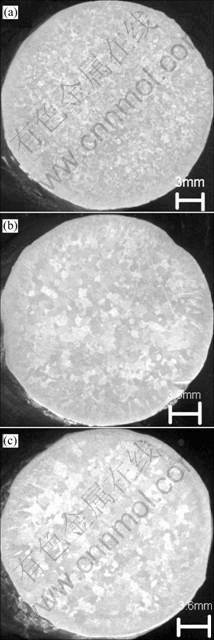
Fig.3 Marcographs of A356 alloys after re-melted for three times
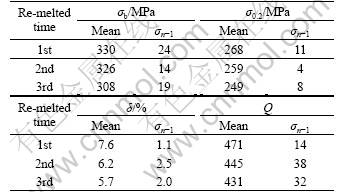
As shown in Fig.4, it can be found that the fracture mechanism appears to be micro-void coalescence (dimples) with obvious plastic tearing mark. As shown in Table 1, A356 alloy can obtain the favorite tensile properties after re-melted for 3 times. This work indicates that A356 alloy can be used for 3 times, maintaining the equiaxial structure and obtaining comparably favorite mechanical properties.
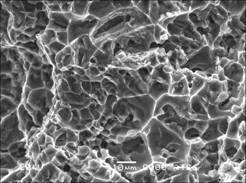
Fig.4 SEM image of fracture surface of A356 alloy
Table 1 Mechanical properties of samples vs re-melted times
However, it also shows that silicon could decrease the grain-refining efficiency of TiAl3. The formation of Ti(Al3-xSix) may yield the instability of TiAl3 phase when mixed with silicon. The recent work[5-6] showed that when the Al-based alloy with boron together with titanium can be electrolyzed in the industrial electrolyzer, the grain refinement efficiency would dramatically increase.
4 Conclusions
The directly electrolyzed Al-based alloy could be re-melted for six times, keeping the favorite grain-refining persistency. Producing the Al-based alloy by electrolysis provides a practical and efficient self-refined route. This Al-based alloy can be used as the starting material for producing A356 alloy in the normal practical application. More work should be done on electrolyzing the Al-based alloy with titanium and other elements, such as boron.
References
[1] MOHANTYY P S, GRUZLESKI J E. Mechanism of grain refinement in aluminium [J]. Acta Metall Mater, 1995, 43(5): 2001-2012.
[2] EASTON M A, STJOKN D H. A model of grain refinement in incorporating alloy constitution and potency of heterogeneous nucleant particles [J]. Acta Mater, 2001, 49: 1867-1878.
[3] LIU Zhi-yong, WANG Ming-xing, WENG Yong-gang, SONG Tian-fu, XIE Jing-pei and HUO Yu-ping. Grain refinement effects of Al-based alloys with low titanium content produced by electrolysis [J]. Trans Nonferrous Met Soc China, 2002, 12(6): 1121-1126.
[4] LIU Zhi-yong, WANG Ming-xing, WENG Yong-gang, SONG Tian-fu, HUO Yu-ping, XIE Jing-pei. Influence of of silicon content on grain refinement and nucleation growth of Al-based alloys produced by electrolysis [J]. Materials Transactions, Jom, 2003, 44(10): 2157-2162.
[5] WANG Ming-xing, WANG San-jun, LIU Zhi-yong, LIU Zhong-xia, SONG Tian-fu, ZUO Xiu-rong. Effect of B/Ti mass ratio on grain refining of Low-titanium aluminum produced by electrolysis [J]. Materials Science and Engineering A, 2006, A416(1-2): 312-316.
[6] WANG Chun-lei, WANG Ming-xing, LIU Zhi-yong, LIU Zhong-xia, WENG Yong-gang, SONG Tian-fu, YANG Sheng. The grain-refining action of fine TiB2 particles in the electrolytic low-titanium aluminum with Al-Ti-B addition [J]. Mater Sci Eng A, 2006, A427(1/2): 148-153.
[7] LIU Zhi-yong. Grain Refinement Effect and Refinement Mechanism of the Industrial Electrolyzed Al-Based Alloy and its Application [D]. Zhengzhou: Zhengzhou University, 2003: 35-37. (in Chinese)
[8] LIU Xiang-fa, QI Guang-hui, BIAN Xiu-fang. The size sudden change and structure evolvement of the macro-grains of Al-Si alloys [J]. Mater Sci Forum, 2000, 331/337: 367-372.
[9] LIU Zhi-yong, WANG Ming-xing, WENG Yong-gang, SONG Tian-fu, HUO Yu-ping. Crystal nucleation and growth of Al-based alloys produced by electrolysis [J]. Journal of Materials Science and Technology, 2003, 19(5): 427-430.
[10] KORI S A, MURRY B S, CHAKRABORTY M. Development of an efficient grain refiner for Al-7Si alloy [J]. Mater Sci Eng A, 2000, A280: 58-61.
[11] DAHLE A K, ST JOHN D H, ATTAVANICH P, TAOPETCH P. Grain formation in AlSi7Mg0.35 foundry alloy at low superheat [J]. Materials Science Forum, 2000, 331(1): 271-276.
(Edited by HE Xue-feng)
Foundation item: Project(0322020600) supported by the Science and Technology Foundation of Henan Province, China
Corresponding author: LIU Zhi-yong; Tel: +86-371-67767776; E-mail: liuzhiyong@zzu.edu.cn
