Oxygen pressure acid leaching of artificial sphalerite catalyzed by Fe3+/Fe2+ self-precipitation
来源期刊:中南大学学报(英文版)2020年第6期
论文作者:徐志峰 张廷安 田磊 龚傲 吴选高 刘燕 魏奎先 于占良
文章页码:1703 - 1713
Key words:leaching mechanism catalyzed by Fe3+/Fe2+ self-precipitation; potential curves; artificial sphalerite; leaching kinetics; activation energy; reaction orders
Abstract: The mechanism of oxygen pressure acid leaching of sphalerite catalyzed by Fe3+/Fe2+ self-precipitation was investigated in this study. Artificial sphalerite was fabricated with varying amounts of iron content via the sintering of ZnS and FeS and used for the pressure acid leaching experiment. The variations in the potential of the pressure leaching system were investigated by using a self-designed potential autoclave. The results showed that compared to the non-iron sphalerite, there was a violent redox reaction between the 25.70% Fe-artificial sphalerite and dissolved oxygen during the process of pressure leaching; and the catalytic mechanism was attributed to the redox couple Fe3+/Fe2+, where Fe3+oxidizes the H2S gas film and the reduced Fe2+ state is subsequently oxidized by the dissolved oxygen. Furthermore, the effect of temperature, H2SO4 concentration, and oxygen partial pressure on the artificial sphalerite with different iron contents was studied. The sphalerite samples with iron content were observed to dissolve more easily in sulfuric acid compared to the non-iron samples. Moreover, the activation energy of artificial sphalerite was observed to be lower in the sample with 25.70% iron content (22.26 kJ/mol) compared to that with no iron (32.31 kJ/mol); and the apparent reaction orders were obtained with respect to H2SO4 concentration (1.10 and 1.36) and oxygen partial pressure (1.29 and 1.41), respectively. A comprehensive kinetic model was developed on the basis of the experimental data and the fitted leaching ratio plot; and the kinetic equations for the leaching of sphalerite catalyzed by Fe3+/Fe2+ self-precipitation were determined.
Cite this article as: TIAN Lei, GONG Ao, WU Xuan-gao, XU Zhi-feng, ZHANG Ting-an, LIU Yan, WEI Kui-xian, YU Zhan-liang. Oxygen pressure acid leaching of artificial sphalerite catalyzed by Fe3+/Fe2+ self-precipitation [J]. Journal of Central South University, 2020, 27(6): 1703-1713. DOI: https://doi.org/10.1007/s11771-020-4401-3.

J. Cent. South Univ. (2020) 27: 1703-1713
DOI: https://doi.org/10.1007/s11771-020-4401-3

TIAN Lei(田磊)1, GONG Ao(龚傲)1, WU Xuan-gao(吴选高)1, XU Zhi-feng(徐志峰)1,
ZHANG Ting-an(张廷安)3, LIU Yan(刘燕)3, WEI Kui-xian(魏奎先)2, YU Zhan-liang(于占良)2
1. School of Metallurgical and Chemical Engineering, Jiangxi University of Science and Technology,Ganzhou 341000, China;
2. The State Key Laboratory of Pressure Hydrometallurgical Technology of Associated NonferrousMetal Resources, Kunming 650503, China;
3. Key Laboratory for Ecological Utilization of Multimetallic Mineral, Ministry of Education,Shenyang 110819, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: The mechanism of oxygen pressure acid leaching of sphalerite catalyzed by Fe3+/Fe2+ self-precipitation was investigated in this study. Artificial sphalerite was fabricated with varying amounts of iron content via the sintering of ZnS and FeS and used for the pressure acid leaching experiment. The variations in the potential of the pressure leaching system were investigated by using a self-designed potential autoclave. The results showed that compared to the non-iron sphalerite, there was a violent redox reaction between the 25.70% Fe-artificial sphalerite and dissolved oxygen during the process of pressure leaching; and the catalytic mechanism was attributed to the redox couple Fe3+/Fe2+, where Fe3+oxidizes the H2S gas film and the reduced Fe2+ state is subsequently oxidized by the dissolved oxygen. Furthermore, the effect of temperature, H2SO4 concentration, and oxygen partial pressure on the artificial sphalerite with different iron contents was studied. The sphalerite samples with iron content were observed to dissolve more easily in sulfuric acid compared to the non-iron samples. Moreover, the activation energy of artificial sphalerite was observed to be lower in the sample with 25.70% iron content (22.26 kJ/mol) compared to that with no iron (32.31 kJ/mol); and the apparent reaction orders were obtained with respect to H2SO4 concentration (1.10 and 1.36) and oxygen partial pressure (1.29 and 1.41), respectively. A comprehensive kinetic model was developed on the basis of the experimental data and the fitted leaching ratio plot; and the kinetic equations for the leaching of sphalerite catalyzed by Fe3+/Fe2+ self-precipitation were determined.
Key words: leaching mechanism catalyzed by Fe3+/Fe2+ self-precipitation; potential curves; artificial sphalerite; leaching kinetics; activation energy; reaction orders
Cite this article as: TIAN Lei, GONG Ao, WU Xuan-gao, XU Zhi-feng, ZHANG Ting-an, LIU Yan, WEI Kui-xian, YU Zhan-liang. Oxygen pressure acid leaching of artificial sphalerite catalyzed by Fe3+/Fe2+ self-precipitation [J]. Journal of Central South University, 2020, 27(6): 1703-1713. DOI: https://doi.org/10.1007/s11771-020-4401-3.
1 Introduction
Pressure leaching is considered to be the most promising substituted revolution in the Zn production industry, as it causes less pollution and produces a higher percentage of zinc and sulphur extraction [1, 2]. However, the sphalerite has a covalent bond lattice structure, and its solubility product is very low. Therefore, it requires highly acidic conditions along with high pressure to ensure a smooth progress of the reaction during the oxygen pressure leaching process [3, 4]. Thus, there are a lot of issues in the pressure leaching process of sphalerite such as high demand for equipment and large investment [5-7].
As sphalerite is a semiconductor and the leaching process is generally electrochemical in nature, defects and impurities in the lattice structure have a great influence on the kinetics of the electrochemical process [8]. Therefore, impurities, different mineral phases and different ions in the solution are important factors in the leaching process. Moreover, in an oxygen pressure leaching system, the Fe ions and other electron transfer media act as catalysts, which precipitate into the solution under the influence of an acid. The chemical reactions between the ore particles and the catalysts simultaneously occur at the liquid-solid and gas-solid interfaces and the catalysts are quickly regenerated in the gas phase, liquid phase, and gas-liquid interfaces. Thus, the leaching rate is considerably improved [9]. Therefore, it is important to investigate the mechanism of oxygen pressure leaching of sphalerite catalyzed by Fe3+/Fe2+ self-precipitation.
The ongoing research on catalysis in metallurgy has recently brought to light the fact that catalysts can considerably increase the leaching efficiency, shorten the leaching time, and improve the leaching conditions in the mineral leaching process. Therefore, many researchers have intensively studied the influence of catalytic agents on the oxygen pressure leaching of sphalerite: GHOSH et al [10] investigated the kinetics of sphalerite leaching in ammonia using Cu(II) as an oxidation catalyst. The results showed that the surface reaction was the reaction-controlling step. The apparent activation energy was estimated to be 48.3 kJ/mol and the apparent reaction orders of 0.2, 0.3, and 0.3 were obtained with respect to oxygen partial pressure, concentration of ammonia, and Cu(II) concentration, respectively. ZHANG et al [11] studied the leaching process of ZnS in the Mn2+ catalyst system and investigated the factors influencing the leaching ratio of zinc. The optimum operation condition was obtained by an orthogonal experiment, and the leaching ratio of zinc was reportedly more than 95%.
However, in the literatures, no information is available on the mechanism and electrochemistry of oxygen pressure leaching of sphalerite catalyzed by Fe3+/Fe2+ self-precipitation. In this study, to further reveal the catalytic mechanism of Fe in the leaching process, artificial sphalerite with different iron contents was used as the raw material. The potential variations during the oxygen pressure leaching of sphalerite catalyzed by Fe3+/Fe2+ self-precipitation were researched by using a potential autoclave and a kinetic mathematical model for the leaching system was proposed. This investigation can help to identify the conditions required to enhance the leaching reaction, improve productivity and reduce the operation cost.
2 Experiment
2.1 Materials
Artificial sphalerite with different Fe contents was used as the raw material in this study, and was prepared by sintering a certain proportion of FeS and ZnS. The study was conducted under an inert gas atmosphere. The temperature and time of the solid state sintering were 1223 K and 120 min, respectively, and the temperature and time of homogenization were 1123 K and 60 min, respectively. We synthesized sphalerite with different Fe contents (i.e., 0 wt% and 25.70 wt%). The highest iron content that can be achieved in the synthesis of sphalerite is about 26 wt% or 45% (mol) FeS [12]. To better study the catalyzed leaching mechanism of sphalerite with Fe3+/Fe2+ self-precipitation, samples of artificial sphalerite (53-74 μm) with 0 and 25.70 wt% iron contents were used as materials for the oxygen pressure leaching experiment.
The XRD patterns of the artificial sphalerite are shown in Figure 1. Apparently, there is no independent FeS phase, and the main structure is a sphalerite crystal form.

Figure 1 XRD patterns of artificial sphalerite:
2.2 Equipment
The pressure leaching experiment was carried out in the FCFD 2-1.0 potential autoclave (2000 mL). The body of the autoclave was made of zirconium, and the maximum pressure and temperature that can be achieved by the autoclave are 6.0 MPa and 573 K, respectively. A diagrammatic sketch and the physical chart of the FCFD 2-1.0 potential high-pressure autoclave are shown in Figures 2 and 3, respectively.
2.3 Leaching experiment procedure
The flowchart representing the process of the pressure leaching of the artificial sphalerite is shown in Figure 3.

Figure 2 Device attachment of potential autoclave:(1-Oxygen bottles; 2-Heating jacket; 3-Control cabinet; 4-Electrode measuring instrument autoclave; 5-Reference electrode; 6-Measuring electrode; 7-Agitator blade; 8-Agitator blade Heater wire; 9-Oxygen bomb; 10-Feed inlet; 11-Discharge port; 12-Tachometry; 13-Motor; 14-Thermocouple; 15-Agitator blade heater wire)

Figure 3 Flowchart of process of pressurized leaching of artificial sphalerite
1000 mL high purity water and 40.0 g artificial sphalerite were placed in the potential autoclave and the heating device is started. Next, sulfuric acid solutions of different concentrations (200 mL) were pressed into the zirconium autoclave by oxygen and the agitator blade was stirred at 500 r/min. The following chemical equations took place:
ZnS+FeS+2H2SO4=ZnSO4+FeSO4+2H2S (1)
4FeSO4+O2+2H2SO4=2Fe2(SO4)3+2H2O (2)
H2S+Fe2(SO4)3=2FeSO4+S+H2SO4 (3)
2H2S+O2=2S+2H2O (4)
Then, 5 mL of the leaching solution was withdrawn at appropriate time intervals, and the zinc concentration was analyzed by using an inductively coupled plasma (ICP) emission spectrometer (Leeman, USA). The leaching ratio of Zn (α) is calculated by using Eq. (5):
α=(C1V1)/(m0x0)×100% (5)
where C1 is the concentration of Zn in the solution, g/L; V1 is the volume of the solution, L; m0 is the quality of the materials, g; and x0 is the valuable element content, %.
3 Results and discussion
3.1 Effect of Fe content on zinc extraction
Zinc extraction using artificial sphalerite with different iron contents is shown in Figure 4. Apparently, the dissolution of the artificial sphalerite is sensitive to the amount of the Fe content: 96.89% of zinc was extracted from the 25.70% Fe-artificial sphalerite after 90 min of leaching, whereas 58.05% was obtained from the non-iron artificial sphalerite after the same leaching time. Thus, the effect of Fe content in the artificial sphalerite has a significant influence on the zinc leaching ratio.

Figure 4 Effect of Fe content on zinc extractions from artificial sphalerite
3.2 Comparison of electricpotential diagrams
The effect of system potential on the non-iron artificial sphalerite and that with 25.70 wt% iron was investigated. The reaction temperature was 403 K; H2SO4 concentration was 110 g/L; oxygen partial pressure was 0.8 MPa; agitation rate was 500 r/min; and the liquid-solid ratio was 30:1. Besides, 0.4 g of calcium lignosulfonate was added to the system. The changes in the relative potential during the leaching process are shown in Figure 5.
In Figure 5(a), the curve of the relative potential of the artificial sphalerite with no iron is divided into three areas. The following observations are made: 1) After adding sulfuric acid and oxygen, the system potential sharply increases. Although a part of the H+ is consumed and produces H2S, the initial leaching reaction is still weak compared with that of the 25.70% Fe-artificial sphalerite. Therefore, the dissolved oxygen cannot be consumed in large quantities. Since consumption of the H2S gas film is the dynamic basis of the continuous leaching process, and it is difficult to generate elemental S and destroy the H2S gas film in the absence of the redox couple Fe3+/Fe2+ that functions as the electron transfer carrier, there are no fluctuations in the system potential. 2) At the beginning of the leaching process, H2S is slowly oxidized by the dissolved oxygen, and the potential of the system gradually increases. 3) At the final stage of the leaching process, the basic reaction reaches its equilibrium. Therefore, there is no change in the potential of the system.

Figure 5 Changes in relative potential during leaching process:
In Figure 5(b), the curve, representing the relative potential of the 25.70% Fe-artificial sphaleriteis, was divided into four areas, and the following observation are made: 1) After adding sulfuric acid and oxygen, the system potential increases rapidly at first and then decreases. This phenomenon is due to the addition of considerable quantities of hydrogen ions and oxygen, which rapidly increases the potential of the leaching system.Subsequently, the sphalerite in the pyrolysis activation state causes a preliminary and rapid acid dissolution. While on one hand, a certain amount of H2S is generated, which causes the sulfuric acid to be consumed; on the other hand, the Fe2+ ions, leached from the mineral particles are rapidly oxidized by the dissolved oxygen, which further reduces the potential. 2) At the beginning of the leaching process, the system potential shows an overall upward trend with denticle-shaped fluctuations (as shown in region A in Figure 6(a)), which is caused by the redox couple Fe3+/Fe2+, mainly after the precipitation of Fe2+ from the original mineral. The redox couple Fe3+/Fe2+ improves also effectively the oxidation and elimination of the H2S gas film. 3) During the middle stage of the leaching process, the potential continues to rise, but the potential fluctuations are lower compared to the previous stage because the sphalerite has been consumed. Therefore, more number of S2- and Fe2+ ions are oxidized and leached, thereby increasing the system potential. Eventually, the presence of a large number of Fe3+ ions and the reduction of the S2- ions gradually weaken the fluctuations in the electric potential and the curve shows a smooth upward trend. 4) At the final stage of the leaching process, since the basic leaching reaction has reached its equilibrium, and the Zn leaching ratio of more than 90% is achieved in 70 min, no significant change is observed in the potential of the system.

Figure 6 Comparison of leaching potentials of artificial sphalerite (leaching time: 20-60 min):
The leaching potentials of the artificial sphalerite with no iron and that with 25.70% iron content were compared (leaching time: 20-60 min), and the potential curves were fitted. The result is shown in Figure 6.
Figure 6 shows that the slope (5.09) of the leaching potential curve for the 25.70% Fe-artificial sphaleriteis, which is much higher than that of the non-iron artificial sphalerite. Further combined with Table 1, the results indicate that the redox reaction with the 25.70% Fe-artificial sphaleriteis is very intense and rapid. Thus, we conclude that the redox couple Fe3+/Fe2+ can effectively promote the zinc leaching ratio.
Table 1 Zinc leaching ratio of artificial sphalerite with no iron and 25.70% iron content

3.3 Analysis of kinetic equation
Figure 7 shows SEM micrographs of the artificial sphalerite samples.
The micrographs in the figure show the surface of the artificial sphalerite is dense, without fine pores, and covered by fine particles. The leaching data in Figures 8(a) and (b) were fitted, and the results showed that the interface dynamic equation [13] had the optimal linear correlation. Therefore, the equation is used to analyze the acid leaching behavior of artificial sphalerite samples.
In the leaching experiment, the dense solid product layer of S can be effectively destroyed by adding calcium lignosulfonate [14-16]. Therefore, compared to the other steps,the resistance of the layer can be considered to be relatively small. When the leaching process is controlled by the chemical reaction, or by a hybrid control, which is explained in detail later in this section, the equation can be expressed as follows [17]:
1-(1-α)1/3=KCn1Pn2exp[(-E/(RT)]/(r0ρ) (6)
K=k1k2/(k1+k2) (7)
where α is the Zn leaching ratio, %; K is the integrated rate constant; k1 is the chemical rate constant; k2 is the external diffusion rate constant; t is the leaching time, min; C is the concentration of Zn in the solution, g/L; P is the partial pressure of oxygen, MPa; M is the additive amount of catalyst, mol/L; r0 is the original radius of the particles, μm; T is the reaction temperature, K; ρ is the density of the particles, kg/m3; R is the gas constant,8.314 J/(mol·K); E is the apparent activation energy, kJ/mol; and ni is the order of the reaction.

Figure 7 SEM patterns of artificial sphalerite:
When k1>>k2, the leaching process is completely controlled by the gas film diffusion; when k2>>k1, the leaching process is completely controlled by the interfacial chemical reaction; and when k1≈k2, the leaching process is controlled to some extent by both, and is termed as a hybrid control.
3.4 Leaching experiment and kinetic data calculation
3.4.1 Calculation of activation energy
The effect of the reaction temperature on the two artificial sphalerite samples is presented under the following conditions: the oxygen partial pressure is 0.8 MPa; H2SO4 concentration is 110 g/L; the agitation rate is 500 r/min; the liquid- to-solid ratio is 30:1; and the amount of calcium lignosulfonate added is 0.4 g. The experimental data and the fitting results are shown in Figure 8.
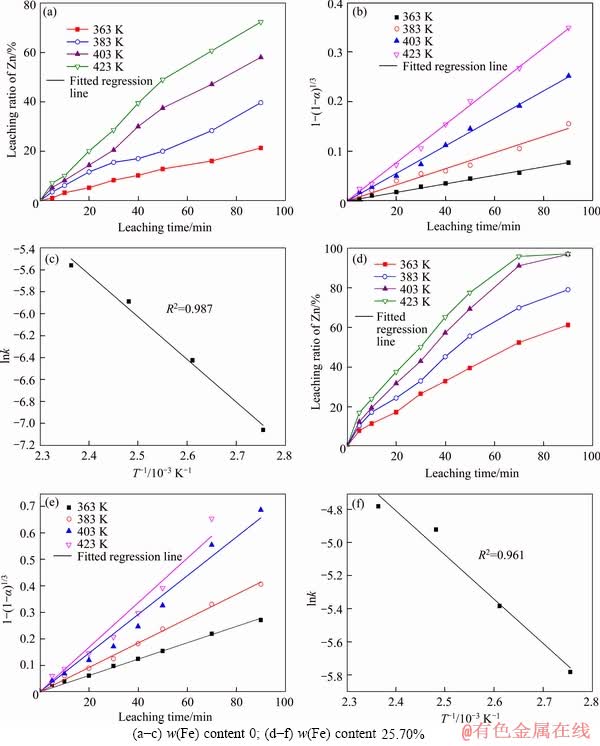
Figure 8 Effect of reaction temperature on leaching of artificial sphalerite:
The fitting results are shown in Figures 8(b) and (e); and the plots of the reciprocal of the reaction temperature (T-1) versus the natural logarithm of the apparent rate constant (lnk), i.e., the Arrhenius plots are shown in Figures 8(c) and (f). The apparent activation energy is determined by Eq. (8):
k=A0exp[-E/(RT)] (8)
where k is the reaction rate constant; T is the reaction temperature, K; and A0 is the pre-exponential factor.
The activation energy of artificial sphalerite was observed to be lower in the sample with 25.70% iron content (22.26 kJ/mol) compared to that with no iron (32.31 kJ/mol). This indicates that the oxygen pressure acid leaching of the artificial sphalerite was hybrid controlled, and the iron in the sphalerite accelerates the leaching efficiency.
The leaching kinetic equation describing the effect of temperature on the 25.70% Fe-artificial sphalerite is shown as follows:
lnk=1.62-2.68×103/T (9)
3.4.2 Calculation of reaction order
The influences of the H2SO4 concentration on the zinc leaching ratios of the 0 and 25.70 wt% Fe-artificial sphalerites were examined in the range of 50 to 140 g/L under constant conditions. The experimental data and the fitting results are shown in Figure 9.
Figures 9(a) and (d) show the leaching rate of zinc at different H2SO4 concentrations during pressure leaching. Figures 9(b) and (e) show the fitting results and the natural logarithm of the H2SO4 concentration (ln[H2SO4]) versus the plot of the natural logarithm of the apparent rate constant (lnk) is shown in Figures 9(c) and (f). The order of the dissolution of the 0 and 25.70% Fe-artificial sphalerite with respect to the H2SO4 concentration is obtained using the plot in Figures 9(c) and (f). The reaction orders for zinc extraction calculated from Figures 9(c) and (f) were 1.36 and 1.10, respectively, and the leaching kinetic equation describing the effect of H2SO4 concentration on the 25.70% Fe-artificial sphalerite is shown as follows:
lnk=-12.27+1.36ln[H2SO4] (10)
lnk=-10.49+1.10ln[H2SO4] (11)
Next, the influences of oxygen partial pressure on the zinc leaching ratio of the 0 and 25.70% Fe-artificial sphalerite were examined in the range of 0.4 to 1.0 MPa under constant conditions. The experimental data and the fitting results are shown in Figure 10.

Figure 9 Effect of sulfuric acid concentration on leaching of artificial sphalerite:
Figures 10(a) and (d) show the leaching rate of zinc at different the oxygen partial pressures during pressure leaching. The fitting results are shown in Figures 10(b) and (e) and the natural logarithm of the oxygen partial pressure (lnPO2) versus the plot of the natural logarithm of the apparent rate constant (lnk) is shown in Figures 10(c) and (f).

Figure 10 Effect of oxygen partial pressure on leaching of artificial sphalerite:
The reaction order for the dissolution of the 0 and 25.70 wt% Fe-artificial sphalerite was found proportional to 1.41 and 1.29 power of the oxygen partial pressure. Thus, the leaching kinetic equation describing the effect of oxygen partial pressure on the leaching of the 25.70% Fe-artificial sphalerite is shown as follows:
lnk=-5.55+1.41lnPO2 (12)
lnk=-4.70+1.29lnPO2 (13)
3.4.3 Establishment of kinetic equation
Based on Eqs. (6) and (7), the kinetic equation can be expressed as:
1-(1-α)1/3=K0×[H2SO4] n1×PO2n2×exp[-E/(RT)]×t (14)
where K0 is the pre-exponential factor.
The relationship between 1-(1-α)1/3 and [H2SO4]n1×PO2n2×exp[-E/(RT)]×t of the 0 and 25.70% Fe-artificial sphalerite is illustrated in Figure 11. The straight line can be fitted to the data with a correlation coefficient over 0.97. Thus, from Figure 11, K0 values are determined to be 1.60×10-3 and 5.04 ×10-4.
Based on the E, n1, n2 and K0 values, the kinetic equation for the oxygen pressure acid leaching of 0 and 25.70% Fe-artificial sphalerite can be expressed as:
1-(1-α)1/3=1.60×10-3×[H2SO4]1.36PO21.41×
exp[-32310/(RT)]×t (15)
1-(1-α)1/3=5.04×10-4×[H2SO4]1.10PO21.29×
exp[-22260/(RT)]×t (16)
4 Conclusions
1) Compared to the non-iron sphalerite, changes in the relative potential of the 25.70% Fe-artificial sphalerite on oxygen leaching were observed to be more intense, which shows that the redox reaction in the latter was very intense and rapid, and the redox couple Fe3+/Fe2+ can effectively promote the zinc leaching ratio.
2) The effects of iron content, temperature, H2SO4 concentration, and oxygen partial pressure on the pressure leaching of artificial sphalerite with different iron contents were studied and the results showed that all these parameters have significant influence on the oxygen pressure acid leaching of artificial sphalerite. For the oxygen pressure acid leaching of 25.70% Fe-artificial sphalerite, the E, n1 and n2 were determined to be 22.26 kJ/mol, 1.10, and 1.29, respectively. The artificial sphalerite was observed to be lower in the sample with 25.70% iron content (22.26 kJ/mol) compared to that with no iron (32.31 kJ/mol), which means that the oxygen pressure acid leaching of artificial sphalerite was hybrid controlled. This indicates that the iron in the sphalerite accelerates the leaching efficiency, and the leaching reaction becomes less sensitive to temperature. Finally, the kinetic equations for fitting the results were determined as:
1-(1-α)1/3=1.60×10-3×[H2SO4]1.36PO21.41×
exp[-32310/(RT)]×t
1-(1-α)1/3=5.04×10-4×[H2SO4]1.10PO21.29×
exp[-22260/(RT)]×t

Figure 11 Relationship between 1-(1-α)1/3 and [H2SO4]n1×PO2n2×exp[-E/(RT)]×t:
References
[1] TIAN Lei, LIU Yan, ZHANG Ting-an, LV Guo-zhi, ZHOU Shuang, ZHANG Guo-quan. Kinetics of indium dissolution from marmatite with high indium content in pressure acid leaching [J]. Rare Metals, 2017, 36(1): 69-76. DOI: DOI: 10.1007/s12598-016-0762-z.
[2] GU Yan, ZHANG Ting-an, LIU Yan, MU Wang-zhong, ZHANG Wei-guang, DOU Zhi-he, JIANG Xiao-li. Pressure acid leaching of zinc sulfide concentrate [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 136-140. DOI: 10.1016/s1003-6326(10)60028-3.
[3] ANDREI B, NICOLAE B, GHEORGHE D, VIORICA V, ANDREI IONUT A. The determination of the Fe content in natural sphalerites by means of Raman spectroscopy [J]. Vibrational Spectroscopy, 2013, 68: 220-224. DOI: 10.1016/ j.vibspec.2013.08.007.
[4] ZHANG Yan-juan, LI Xuan-hai, PAN Liu-ping, WEI Yan-song, LIANG Xin-yuan. Effect of mechanical activation on the kinetics of extracting indium from indium-bearing zinc ferrite [J]. Hydrometallurgy, 2010, 102(1-4): 95-100. DOI: 10.1016/j.hydromet.2010.02.003.
[5] TIAN Lei, XU Zhi-feng, CHEN Li-jie, LIU Yan, ZHANG Ting-an. Study on oxygen gas holdup and kinetics using various types of paddles during marmatite leaching process [J]. Hydrometallurgy, 2018, 180: 158-171. DOI: 10.1016/ j.hydromet.2018.06.011.
[6] XIE Ke-qiang, YANG Xian-wan, WANG Ji-kun, YAN Jiang-feng, SHEN Qing-feng. Kinetic study on pressure leaching of high iron sphalerite concentrate [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(1): 187-194. DOI: 10.1016/S1003-6326(07)60070-3.
[7] XU Zhi-feng, JIANG Qing-zheng, WANG Cheng-yan. Behavior of zinc sulfur and iron in low-temperature pressure leaching process of marmatite [J]. Nonferrous Metals (Extractive Metallurgy), 2012(2): 6-11. DOI: 10.3969/j.issn. 1007-7545.2012.07.002. (in Chinese)
[8] LIU Jian, EJTEMAEI Majid, NGUYEN Anh V, WEN Shu-ming, ZENG Yong. Surface chemistry of Pb-activated sphalerite [J]. Minerals Engineering, 2020, 145: 106058. DOI: 10.1016/j.mineng.2019.106058.
[9] FOMCHENKO N V, MURAVYOV M I. Effect of sulfide mineral content in copper-zinc concentrates on the rate of leaching of non-ferrous metals by biogenic ferric iron [J]. Hydrometallurgy, 2019: 82-87. DOI: 10.1016/j.hydromet. 2019.02.002.
[10] GHOSH M K, DAS R P, BISWAS A K. Oxidative ammonia leaching of sphalerite Part II: Cu(II)-catalyzed kinetics [J]. International Journal of Mineral Processing, 2003, 70(1-4): 221-234. DOI: 10.1016/S0301-7516(03)00024-3.
[11] ZHANG Huai-wei, WANG Ji-kun, LIANG Duo-qiang. Study of pressure leaching of zinc sulfide in Mn2+ catalyst system [J]. Mining & Metallurgy, 2008, 17(2): 51-54. DOI: CNKI:SUN:KYZZ.0.2008-02-013. (in Chinese)
[12] XU Zhi-feng, QIU Ding-fan, WANG Hai-bei. Research on synthesis of marmatite and thermodynamics of marmatite in leaching [J]. The Chinese Journal of Process Engineering, 2006(6): 1-7. DOI: 10.3321/j.issn:1009-606X.2006. z1.001. (in Chinese)
[13] TAPIO S, HENRIK G, HEIDI B, JOHAN W, DMITRY Y M. Mechanistic modelling of kinetics and mass transfer for a solid-liquid system: Leaching of zinc with ferric iron [J]. Chemical Engineering Science, 2010, 65(15): 4460-4471. DOI: 10.1016/j.ces.2010.04.004.
[14] TIAN Lei, ZHANG Ting-an, LIU Yan, LV Guo-zhi, TANG Jun-jie. Oxidative acid leaching of mechanically activated sphalerite [J]. Canadian Metallurgical Quarterly, 2018, 57(1): 59-69. DOI: 10.1080/00084433.2017.1367884.
[15] TIAN Lei, LIU Yan, LV Guo-zhi, YU Xiong, ZHOU Shuang, ZHANG Ting-an. Research on sulphur conversion and acid balance from marmatite in pressure acid leaching [J]. Canadian Metallurgical Quarterly, 2016, 55(4): 438-447. DOI: 10.1080/00084433.2016.1210274.
[16] FAN Yang-yang, LIU Yan, NIU Li-ping, JING Ting-le, ZHANG Ting-an. Separation and purification of elemental sulfur from sphalerite concentrate direct leaching residue by liquid paraffin [J]. Hydrometallurgy, 2019, 186: 162-169. DOI: 10.1016/j.hydromet.2019.04.009.
[17] LI Hong-gui. Science of hydrometallurgy [M]. Changsha: Central South University Press, 2002. (in Chinese)
(Edited by ZHENG Yu-tong)
中文导读
Fe3+/Fe2+自沉淀催化人工闪锌矿的氧压酸浸
摘要:本文研究了Fe3+/Fe2+自沉淀催化闪锌矿氧压酸浸的机理。利用ZnS和FeS烧结制备了具有不同铁含量的人造闪锌矿,并将其用于加压酸浸实验。利用自行设计的电位高压釜,研究了压力浸出系统的电势变化。结果表明,与不含铁闪锌矿相比,加压浸出过程中含25.70%铁的人造闪锌矿与溶解氧之间发生剧烈的氧化还原反应。催化机理归因于氧化还原对Fe3+/Fe2 +,其中Fe3 +氧化H2S气膜,还原的Fe2 +随后被溶解氧氧化。此外,研究了温度、H2SO4浓度和氧分压对不同铁含量的人造闪锌矿的影响。与不含铁的样品相比,含铁的闪锌矿样品更容易溶于硫酸。铁含量为25.70% (22.26 kJ/mol)的人工闪锌矿的活化能比不含铁样品的活化能低(32.31 kJ/mol),并分别得到H2SO4浓度(1.10和1.36)和氧分压(1.29和1.41)的表观反应级数。根据实验数据和拟合的动力学图谱,建立了相关的动力学模型,并得到了Fe3+/Fe2+自沉淀催化闪锌矿浸出的动力学方程。
关键词:Fe3+/Fe2+自沉淀催化浸出机理;电势曲线;人造闪锌矿;浸出动力学;活化能;反应级数
These authors contribute equally: TIAN Lei, GONG Ao.
Foundation item: Projects(51804136, 51764016) supported by the National Natural Science Foundation of China; Project(U1402271) supported by the Joint Funds of the National Natural Science Foundation of China; Project(20181BAB216017) supported by the Jiangxi Provincial Natural Science Foundation, China; Project(GK-201803) supported by the Research Fund Program of State Key Laboratory of Rare Metals Separation and Comprehensive Utilization, China; Projects(yy2016001, yy2016012) supported by the Research Fund Program of the State Key Laboratory of Pressure Hydrometallurgical Technology of Associated Nonferrous Metal Resources, China
Received date: 2020-01-10; Accepted date: 2020-03-18
Corresponding author: XU Zhi-feng, PhD, Professor; Tel: +86-13407078998; E-mail: xzf_1@163.com; ORCID: 0000-0003-0851-9598; ZHANG Ting-an, PhD, Professor; Tel: +86-13840205331; E-mail: zta2000@163.net; ORCID: 0000-0002-9934- 4713

