Trans. Nonferrous Met. Soc. China 23(2013) 2781-2785
Extraction of aluminum from alumina by disproportionation process of AlCl in vacuum
Yue-bin FENG1, Qing-chun YU2, Bin YANG2, Yong-nian DAI2
1. Faculty of Science, Kunming University of Science and Technology, Kunming 650500, China;
2. National Engineering Laboratory of Vacuum Metallurgy, Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China
Received 16 July 2012; accepted 10 December 2012
Abstract: The extraction conditions of aluminum by the disproportionation process of AlCl in vacuum were investigated using alumina and graphite as raw materials, including reaction temperature, pre-reaction and condenser structure. The results show that the extent of the reaction between alumina and carbon increases with increasing reaction temperature at 1643-1843 K; however, the extraction rate of aluminum increases firstly, and reaches the highest at 1743 K, and then decreases with rise in reaction temperature. The pre-reaction of alumina and carbon increases the extraction rate of aluminum. The impurities C, Al4C3 and Al2O3 in the aluminum product are reduced with reducing the contact surface of the aluminum with CO and with decreasing the condensation temperature, depending on the structure of the condenser.
Key words: aluminum; AlCl; alumina; extraction; vacuum; disproportionation
1 Introduction
Although aluminum is widely distributed in nature, its prices remain high. The main reason is that the cost of the traditional Hall-Héroult process used generally in industry is high. Therefore, the alternative processes are continuously being explored in order to cut the aluminum production cost.
The carbothermal reduction of alumina to produce aluminum has the most possibility to replace the Hall-Héroult process due to its simple procedures and potential to reduce the cost [1,2]. However, it still remains a formidable technical challenge due to the high temperature required (2300-2500 K) and the difficulty in separating aluminum product from the molten residues [3-7]. The disproportionation process of AlCl in vacuum developed from the direct carbothermal reduction process could overcome the above problems, in which the required temperatures are lower and the aluminum product is apart from the residues [8,9].
WANG et al [9] attempted to extract aluminum from bauxite using the disproportionation process of AlCl in vacuum. The extraction rate and the purity of the aluminum product were not satisfying. The research on the behaviors of some additives in the process demonstrated that the extraction rate of aluminum was improved by the additions of Fe2O3 and TiO2, and reduced by the addition of SiO2, and the purity of aluminum was not affected by them [10,11].
The mechanism of the process has been explored. YUAN et al [12,13] and YU et al [14] proposed that Al4O4C and Al4C3 formed from the carbothermal reduction of alumina were chlorinated to AlCl, and AlCl decomposed to Al and AlCl3(g). FENG et al [15] suggested that AlCl was mainly generated from the chlorination of Al2O and Al formed from the carbothermal reduction of aluminum, and Al2O, and Al could also react with CO to form Al4O4C and Al4C3 as side products; however, FENG [16] agreed that Al4O4C and Al4C3 can also be chlorinated to AlCl. Thus it can be inferred that the pre-reaction of alumina and carbon could be an important factor affecting the extraction rate of aluminum.
The purpose of this work is to preliminarily investigate the effects of reaction temperature, pre-reaction and condenser structure on the extraction rate and the purity of aluminum.
2 Experimental
2.1 Apparatus
Experiments were carried out in a furnace designed by ourselves, described in detail in Ref. [17]. The condensers used in this work are shown in Fig. 1.
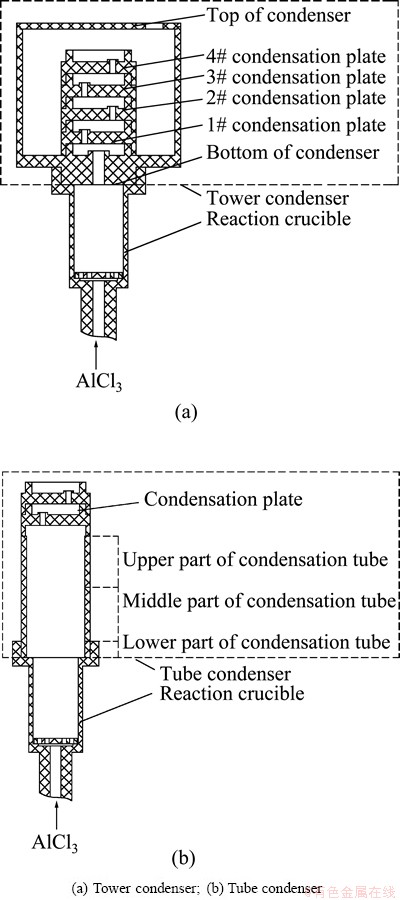
Fig. 1 Schematic diagram of condensers
2.2 Experimental procedure
Alumina (analytical grade) and graphite (fixed carbon content of 99.85%) with molar ratio 1:3 were thoroughly mixed and pressed in a closed die of 20 mm in diameter under 2 MPa to produce cylindrical pellets with mass of about 5 g.
20 g pellets were held in the reaction crucible placed in the furnace. When the pressure in the furnace was pumped to below 5 Pa, the reaction crucible was heated to a certain temperature, and then aluminum chloride anhydrous (analytical grade) was heated to sublime into the crucible. The temperature of the crucible was maintained for a certain time, and then heating was stopped. When the furnace was cooled to room temperature, the residues and the condensates were collected and weighed. The condensate samples were characterized by XRD (D/max-3B).
3 Results and discussion
3.1 Effects of reaction temperature on extraction of aluminum
The tower condenser was used in the experiments, and reaction time was 1 h. The results are shown in Table 1. The residues consisted of unreacted alumina and graphite, and their amounts could reflect the extent of the reaction between alumina and carbon. The condensates on the upper wall of the crucible and the bottom of the condenser were formed by the secondary reactions of the Al2O, Al and CO, and their amounts could reflect the extent of the secondary reactions. The condensates in the condenser consisted of metallic aluminum with impurities Al4C3, Al2O3 and C, and their amounts could reflect roughly the extraction rate of aluminum.
Table 1 Effects of reaction temperature on extraction of aluminum
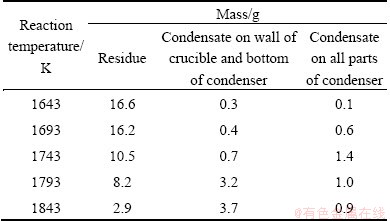
The facts can be seen from Table 1 as follows.
1) The residues were reduced with increasing reaction temperature.
2) The condensates on the upper wall of the crucible and the bottom of the condenser increased with increasing reaction temperature.
3) The condensates in the condenser first increased and then decreased with increasing reaction temperature, and reached the maximum at 1743 K.
The process proceeds through successive steps [15,16]: Firstly, alumina reacts with carbon to generate gaseous Al2O, Al and CO in the crucible; Secondly, the Al2O, Al gases react with AlCl3 to form gaseous AlCl, and react secondarily with CO to form Al4O4C, Al4C3, Al2O3 and C at the same time; Lastly, the AlCl enters condenser and disproportionates to metallic aluminum and gaseous AlCl3. It was deduced that increasing reaction temperature could increase the extent of the reactions between alumina and carbon, but also increase the extent of the secondary reactions between Al2O, Al and CO, thereby resulting in a decrease in AlCl generated from the chlorination of Al2O and Al above 1743 K, and a decrease in the extraction rate of aluminum as a result.
It is concluded that the extent of the reaction between alumina and carbon increased with increasing reaction temperature; however, the extraction rate of aluminum first increased and then decreased with reaction temperature, and reached the highest level at 1743 K due to the secondary reactions between Al2O, Al and CO.
3.2 Effects of pre-reactions on extraction of aluminum
The effects of pre-reactions of alumina and carbon on the extraction of aluminum by the disproportionation process of AlCl in vacuum were investigated through comparative experiments.
Experiment I Alumina and graphite were pre-reacted at 1793 K for 1 h, and then reacted further with AlCl3 gas at the same temperature for 1 h.
Experiment II Alumina and graphite reacted directly with AlCl3 gas at 1793 K for 1 h.
The tower condenser shown in Fig. 1(a) was used in the both experiments, and the results are shown in Table 2.
Table 2 Results of experiments I and II
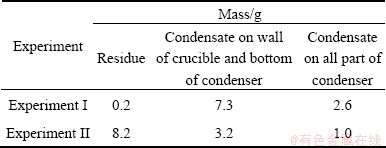
The facts can be seen from Table 2 as follows.
1) The residues in experiment I were less than those in experiment II, because the total time of the reaction between alumina and carbon was 2 h in experiment I, longer than 1 h in experiment II.
2) The condensates on the upper wall of the crucible and the bottom of the condenser in experiment I were more than those in experiment II, because the total time of the reaction between alumina and carbon in experiment I was longer than that in experiment II, resulting in more products formed from the secondary reactions in experiment II.
3) The condensates in the condenser in experiment I were more than those in experiment II under the condition that the chlorination time of the two experiments was equal, namely the extraction rate of aluminum in experiment I was higher than that in experiment II. This fact confirmed again the previous conclusion that the secondary reaction products of Al2O, Al and CO formed from carbothermal reduction of alumina, consisting of Al4O4C, Al4C3, Al2O3 and C, can be also chlorinated to AlCl and further disproportionate to Al and AlCl3[16].
It is concluded that the pre-reactions of alumina and carbon raised the extraction rate of aluminum.
3.3 Effects of condenser structure on purity of aluminum
Aluminum subchloride gas disproportionates to metallic aluminum and gaseous aluminum chloride in the condenser. The metallic aluminum can absorb CO to catalyze its disproportionation to C and CO2, and also react with CO to form Al4C3, Al2O3, C and CO2, resulting in the metallic aluminum containing impurities Al4C3, Al2O3 and C [16]. The extent of detrimental reactions could be related to the structure of condenser.
The effects of condenser structure on the purity of metallic aluminum were investigated through comparative experiments using the tower condenser and the tube condenser shown in Fig. 1.
For the tower condenser, the temperature is decreased in order of 1#, 2#, 3#, 4# condensation plate and the top of the condenser. The bottom part of the tower condenser is thick, and has only one hole in its center for gases entering its chamber, thereby the temperature in the condenser is remarkably lower than that in the crucible. Furthermore, there is only one hole in the condensation plates for gas passing as well so that the temperature difference between them is obvious. Additionally, the metallic aluminum on the condensation plates will have a larger contact surface with CO.
For the tube condenser, the temperature is decreased gradually from the lower part of the condensation tube to the condensation plate; however, it decreases slowly because the condensation tube is not separated from the crucible and each part of it is not separated from each other. The metallic aluminum on the wall of the condensation tube will have a smaller contact surface with CO, compared with the condensation plate.
The comparative experiments were carried out under reaction temperature of 1793 K and reaction time of 1 h, and the results are shown in Table 3 and Table 4.
As can be seen from Table 3, the impurities Al4C3, C and Al2O3 were decreased with decreasing condensation temperature in the tower condenser, and the aluminum with little impurities was obtained on 3#, 4# condensation plate and the top of the condenser with lower temperatures. The results demonstrated that the extents of the disproportionation of CO and the reactions between metallic aluminum and CO, which formed the impurities Al4C3, C and Al2O3, decreased with decreasing temperature.
Table 3 Composition of condensates on different part of tower condenser
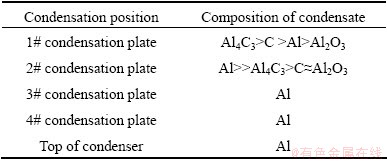
Table 4 Composition of condensates on different parts of tube condenser
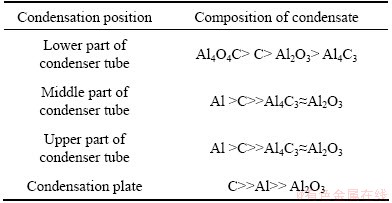
As can be seen from Table 4, the aluminum with few impurities was not obtained in the tube condenser because the temperature could not decrease to low enough for the detrimental reactions ceased. It is worth noticing that the aluminum content in the condensates on the condensation plate with the lowest temperature was lower than that on the wall of the condenser tube. The reason is that the contact surface of the metallic aluminum on the condensation plate with CO was larger than that on the wall of the condensation tube. The other evidence of the view is that the Al4C3, C and Al2O contents in the condensates on 1# condensation plate of the tower condenser were higher than those on the middle part of the tube condenser under the condition that the temperatures of the two positions were approximately equal to the lowest temperature for the disproportionation of AlCl. It is needed to explain that the condensates consisting of Al4O4C, C, Al2O3 and Al4C3 on the lower part of the condensation tube were formed from the secondary reactions of Al-containing gases Al2O, Al and CO.
It is concluded that the structure of condenser should be designed to reduce the condensation temperature and decrease the contact surface of metallic aluminum with CO in order to increase the purity of aluminum product.
4 Conclusions
1) The extent of the reaction between alumina and carbon increases with increasing reaction temperature; however, the extraction rate of aluminum first increases and then decreases with increasing reaction temperature, and reaches the highest level at 1743 K, because the extent of the secondary reaction of Al-containing gases Al2O, Al and CO increases with increasing reaction temperature, resulting in a decrease in the formation of AlCl and the extraction rate of aluminum above 1743 K.
2) The pre-reaction of alumina and carbon increases the extraction rate of aluminum.
3) The structure of condenser should be designed to be beneficial to decrease the condensation temperature and the contact interface of metallic aluminum with CO so that the extents of the disproportionation of CO and the reactions of metallic aluminum with CO could be decreased, thereby reducing the formation of C, Al4C3 and Al2O3 and increasing the purity of aluminum.
References
[1] Kusik C L, Syska A, Mullins J, Vejins V. Techno-economic assessment of a carbothermic alumina reduction process [C]//Proceedings of the 119th TMS Annual Meeting, Light Metals 1990.Warrendale: TMS, 1990: 1021-1034.
[2] Cochran C N. Alternate smelting processes for aluminum: Part I [J]. Light Metal Age, 1987, 45(11-12): 15-20.
[3] ALCOCK C B. Plasma processing of oxide systems in the temperature range 1000-3000 K [J]. Pure and Applied Chemistry, 1980, 52(7): 1817-1827.
[4] WAI C M, HUTCHISON S G. A thermodynamic study of the carbothermic reduction of alumina in plasma [J]. Metallurgical and Materials Transactions B, 1990, 21(2): 406-408.
[5] Halmann M, Frei A, Ateinfeld A. Carbothermal reduction of alumina: Thermochemical equilibrium calculation and experimental investigation [J]. Energy, 2007, 32(12): 2420-2427.
[6] Bruno M J. Aluminum carbothermic technology [R]. Washington: U.S. Department of Energy Golden Field Office, 2004.
[7] Murray J P. Aluminum production using high-temperature solar process heat [J]. Solar Energy, 1999, 66(2): 133-142.
[8] WANG Ping-yan, LIU Mou-sheng, LIU Yun-qi, DAI Yong-nian. Thermodynamic of the process of producing aluminum by carbothermic reduction-chlorination from alumina [J]. Nonferrous Metals: Metallurgy, 2006(6): 23-25. (in Chinese)
[9] WANG Ping-yan, LIU Mou-sheng, DAI Yong-nian. Vacuum metallurgy of Al from bauxite by carbothermic reduction- chlorination [J]. Chinese Journal of Vacuum Science and Technology, 2006, 26(5): 377-380. (in Chinese)
[10] Zhu Fu-long, Yang Bin, Yuan Hai-bin, Yu Qing-chun, Xu Bao-qiang, Dai Yong-nian. Silica behavior in the alumina carbothermic reduction-chlorination process [J]. JOM, 2011, 63(8): 119-122.
[11] YUAN Hai-bin, ZHU Fu-long, YU Qing-chun, LI Qiu-xia, XU Bao-qiang, YANG Bin, DAI Yong-nian. Effects of Fe2O3, SiO2 and TiO2 on aluminum produced by alumina carbothermic reduction— chlorination process in vacuum [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(9): 1836-1842. (in Chinese)
[12] Yuan Hai-bin, Yang Bin, Yu Qing-chun, XU Bao-qiang, ZHU Yu-yan, FENG Yue-bin, DAI Yong-nian. Reaction mechanism of AlCl generated by carbothermic chloride to produce aluminum in vacuum [C]//BA De-chun. Proceeding of 9th Vacuum Metallurgy and Surface Engineering Conference. Beijing: Electronics Industry Press, 2009: 39-45.
[13] YUAN Hai-bin, YANG Bin, XU Bao-qiang, YU Qing-chun, FENG Yue-bin, DAI Yong-nian. Aluminum production by carbothermo- chlorination reduction of alumina in vacuum [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1505-1510.
[14] Yu Wen-zhan, Yang Bin, Zhu Fu-long, Jiang Wen-long, Yu Qin-chun, Xu Bao-qiang. Investigation of chlorination process in aluminum production by carbothermic-chlorination reduction of Al2O3 under vacuum [J]. Vacuum, 2012, 86(8): 1113-1117.
[15] FENG Yue-bin, YANG Bin, DAI Yong-nian. Carbothermal reduction-chlorination-disproportionation of alumina in vacuum [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 215-221.
[16] FENG Yue-bin. Study on the carbothermal reduction and the carbothermal reduction-chlorination-disproportionation of alumina in vacuum [D]. Kunming: Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, 2011: 53-88. (in Chinese)
[17] DAI Yong-nian, FENG Yue-bin, YANG Bin, YANG Bu-zheng, LIU Yong-cheng, XU Bao-qiang, LIU Da-chun, YU Qing-chun, MA Wen-hui, QING Bo. A kind of vacuum reaction furnace: China, 200920111773.1 [P]. 2009-08-06. (in Chinese).
真空氯化亚铝歧化法从氧化铝中提取铝
冯月斌1,郁青春2,杨 斌2,戴永年2
1. 昆明理工大学 理学院,昆明 650500;
2. 昆明理工大学 冶金与能源工程学院,真空冶金国家工程实验室,昆明 650093
摘 要:研究了以氧化铝和石墨为原料真空氯化亚铝歧化法提取铝的条件,包括反应温度、预反应和冷凝器的结构。结果表明:在1643~1843 K的温度范围内,氧化铝与碳的反应程度随着反应温度的升高而提高,但铝的提取率首先随着反应温度的升高而提高,在1743 K时达到最高,继续升高反应温度,铝的提取率反而降低;氧化铝与碳进行预反应可以提高金属铝的提取率;金属铝与CO的接触面积越小、冷凝温度越低,C、Al4C3和Al2O3杂质的含量越低,这取决于冷凝器的结构。
关键词:铝;氯化亚铝;氧化铝;提取;真空;歧化
(Edited by Hua YANG)
Foundation item: Project (51264023) supported by the National Natural Science Foundation of China; Project (KKSY201207016) supported by Yunnan Provincial Science and Technology Department, China
Corresponding author: Yue-bin FENG; Tel: +86-871-5916456; E-mail: fenjys@126.com
DOI: 10.1016/S1003-6326(13)62797-1