Trans. Nonferrous Met. Soc. China 23(2013) 2312-2316
Synthesis and electrochemical properties of Li1.03Co0.1Mn1.9FzO4-z material for lithium-ion batteries
Wen-jing LIU, Yun ZHANG, Fu WANG, Chao LU
College of Materials Science and Engineering, Sichuan University, Chengdu 610064, China
Received 9 September 2012; accepted 17 March 2013
Abstract: Li1.03Co0.10Mn1.90FzO4-z (z=0, 0.05, 0.10, 0.15 and 0.20) cathode materials were synthesized by solid-state reaction using Mn2O3, Li2CO3, Co2O3 and LiF as raw materials. The chemical compositions of Li1.03Co0.1Mn1.9FzO4-z were examined by inductively coupled plasma (ICP) and potentiometric analysis, the effects of F-substitution contents on structure, morphology and electrochemical performance of spinel Li1.03Co0.10Mn1.90O4 were studied by X-ray diffraction (XRD), scanning electron microscopy (SEM) and electrochemical measurements. It is found that the Li1.03 Co0.10Mn1.90FzO4-z samples display a single phase of cubic spinel structure. The lattice parameters increase with the increase of F content when z≤0.10. However, the lattice parameters begin to decrease when F content continues to increase. The results show that an appropriate amount of F substitution for O element with Li+, Co3+ improves discharge capacity and structure stability of the materials. The Li1.03Co0.10Mn1.90F0.15O3.85 sample shows an initial discharge capacity of 111.0 mA·h/g and has capacity retention of 97.0% after 30 cycles at 0.2C.
Key words: cathode materials; solid-state reaction; F-substitution; cycle performance
1 Introduction
In recent years, lithium-manganese spinel LiMn2O4 has been considered one of the most promising positive electrode materials for lithium-ion batteries because of its low cost, abundant reserves, simple synthesis technique, and environmental benign [1,2]. However, lithium-manganese spinel LiMn2O4 also has some disadvantages such as severe capacity fading [3]. The main reason of the occurrence of these disadvantages [4,5] is structural instability in the charged state. In order to improve the electrochemical stability of the spinel LiMn2O4 material, many studies have been directed to ion-substituted compounds to improve structural stability and electrochemical performance of the materials. It is found that doping with Li+, Co3+, Al3+, Cr3+ and F- ions [6-10] could enhance the electrochemical stability and cycling performance of the materials. LU et al [11] have reported lithium-rich Li1.02Mn2O4 having an initial discharge capacity of 120 mA·h/g. However, it has serious capacity fade and remains less than 100 mA·h/g after 50 cycles. LiCoxMn2-xFyO4-y, investigated by XIAO et al [5], demonstrated an initial discharge capacity of 123.5 mA·h/g, and the capacity retention was 92.5% after 20 cycles. LIU et al [12] have reported that LiAlxMn2-xO4-yFy prepared by a sol–gel method has an initial capacity of 115 mA·h/g which only drops to 109 mA·h/g after 50 cycles. Thus, it is believed that the substitution of Co3+, F- ions for Mn and O ions can enhance the initial capacity of Li excess spinel LiMn2O4, and the capacity fading speed can also be reduced.
In this work, Li1.03Mn2O4 was considered to be base compound due to its more lithium content while retaining the same structure as LiMn2O4, thus we have an attempt to solve both the initial capacity and capacity fading problems by co-doping with Co3+ and F- in the base structure. Li1.03Co0.10Mn1.90FzO4-z was prepared by solid-state reaction using Li2CO3, LiF, Co2O3 and Mn2O3 as raw materials. The effects of F-substitution on synthesis, morphology and electrochemical performance were examined by X-ray diffraction (XRD), scanning electron microscopy (SEM) and electrochemical measurements.
2 Experimental
2.1 Preparation of spinel Li1.03Co0.10Mn1.90FzO4-z
The mixtures consisting of stoichiometric ratio of Li1.03Co0.10Mn1.90FzO4-z (z=0, 0.05, 0.10, 0.15 and 0.20) using Li2CO3, LiF, Co2O3 and Mn2O3 as raw materials were ball milled for 2 h with ethanol as dispersant. Then the milled powders were dried at 80 °C. The well-mixed mixtures with different contents of F-substitution were initially heated at 500 °C for 4 h, cooled to the temperature, and re-grind again. Then, the materials were calcined at 800 °C for 12 h in air, cooled to room temperature slowly, and Li1.03Co0.10Mn1.90FzO4-z cathode samples were obtained. Li2CO3 and Co2O3 with the purity of 99.0% are of merchant battery grade. LiF used in the experiment was analytical reagent. Mn2O3 was prepared by calcining MnO2 of merchant electronic grade at 700 °C for 12 h in air.
2.2 Characterization
The contents of Li, Co and Mn elements were examined by inductively coupled plasma (ICP), and content of F was examined by potentiometric analysis. The structures of the Li1.03Co0.10Mn1.90FzO4-z samples were detected by X-ray powder diffraction analysis using Cu Kα radiation at room temperature, 40 kV and 25 mA, and the diffraction angle ranged from 10° to 90° with a continuous scanning step of 0.02 (°)/s. The morphology and size of the samples were observed using a scanning electron microscope (JSM-5900 Japan).
2.3 Electrochemical measurement
With lithium metal pieces as the negative electrode, the positive electrode was prepared by mixing 85% active materials, 10% acetylene black (Alfa), and 5% polyvinylidene difuoride (PVDF, Solvay) binder, and N-methyl-2-pyrrolidine (NMP, Alfa) as solvent. The mixed cathode slurry was evenly coated on aluminum foil, then pressed at 20 MPa and dried at 120 °C for 20 h in a vacuum oven. The cells were assembled in an argon-filled glove box using lithium foil as anode, Celgard2400 as separator and 1 mol/L LiPF6 dissolved in a mixture of EC and DMC (1:1) as electrolyte. The charge/discharge tests were performed on a neware battery program control instrument through constant current/constant voltage charge and in constant current discharge in voltage range of 3.0-4.3 V at room temperature.
3 Results and discussion
3.1 X-ray diffraction analysis
Figure 1 shows the X-ray diffraction patterns of Li1.03Co0.10Mn1.90FzO4-z. It can be seen that all the four materials doped with F- exhibit the cubic spinel structure of space group Fd3m, demonstrating that F- are incorporated into the spinel structure. These show that Li1.03Co0.10Mn1.90FzO4-z materials prepared by solid-state method have a well spinel structure. But from Fig. 1, it can be seen that Li1.03Co0.10Mn1.90F0.15O3.85 and Li1.03Co0.10Mn1.90F3.80O0.20 show a MnF3 impurity diffraction. From Table 1, it can be seen that the diffraction angle moves to the lower angle with the increase of F- content. When F-substitution is 15% (mole fraction, the same below if not mentioned), the shift extent of the diffraction peak is reduced, which is consistent with the changes of the lattice parameters in Table 2. The correspondent peaks of lattice planes (222), (400), (331), (440) become more and more acute, which shows that the particle size tends to increase with F- doping. When the amount of F-substitution is 15% (mole fraction), the peak of the sample is the acutest and the peak intensity reaches the maximum, demonstrating that the sample with 15% of F-substitution has the best crystalline. This is consistent with the results of the FWHM and diffraction angle analyses in Table 2.
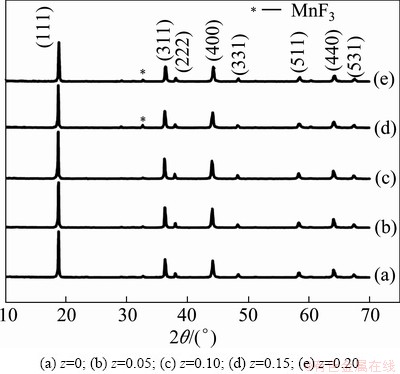
Fig. 1 XRD patterns of prepared Li1.03Co0.10Mn1.90FzO4-z materials
Table 1 Relationship between (311) diffraction angle and F content

Table 2 Relationship among lattice parameter, (111) FWHM and F content
As shown in Table 2, with the substitution of F for O in Co-doped and Li-doped lithium manganese oxide, the lattice parameters gradually increase with the molar ratio of F element increasing from 0 to 0.10. However, when the amount of F-substitution is more than 10%, the lattice parameters begin to decrease with the increase of F- content. The incorporation of fluorine has two effects on the materials. On one hand, O ions are replaced by F-, which makes the amount of Mn3+ increase. Since the radium of the Mn3+ is larger than that of the Mn4+, the incorporation of F- causes a part of oxygen defects in crystal, which make the lattice parameters increase. On the other hand, the electronegativity of F- is larger than that of O ion and σ (Mn—O) enhances due to F- incorporating [13], which makes the lattice parameters decrease. Thereby, in the case of a small amount of F-substitution, the amount of Mn3+ plays a leading role, which makes the lattice parameters increase. However, with more F- incorporated to the materials, the strong electronegativity of F- will play a leading role, which makes the lattice parameters decrease gradually. The expansion of the crystal lattice, which provides more lattice space, is conducive to lithium intercalation and deintercalation, but disadvantageous to the stability of the structure of the material. The small crystal lattice is helpful to the stability of the structure of lithium manganese oxide, but prejudices lithium intercalation and deintercalation in charge/discharge. Table 2 reports that FWHM value is minimum at z=0.15. This demonstrates that crystallinity of the sample, which plays an important role in electrochemical performance, is the best at this moment. When z=0.20, FWHM is more than 0.200, the crystal form of the sample is incomplete, which will be detrimental to the battery cycle performance. This is consistent with SEM. From the ICP analysis and potentiometric analysis, it was confirmed that the chemical compositions of the prepared powders were stoichiometric.
3.2 Morphology
The morphologies of the F-substituted spinel Li1.03Co0.10Mn2O4 are presented in Fig. 2. As can be seen from the figure, the particles of F-substituted samples have uniform size dispersion and unobvious reunion.
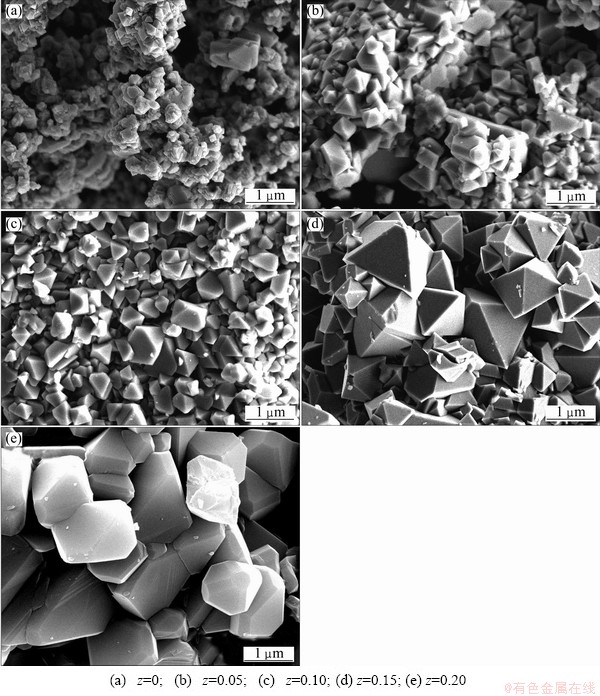
Fig. 2 SEM images of Li1.03Co0.10Mn1.90FzO4-z materials
Comparing with undoped-Li1.03Co0.10Mn1.9O4, the F-substitution samples have favorable spinel octahedral structure, which agrees with XRD. It can also be observed that when z=0.15, the sample has the particles with smooth surface, angular grain, the most perfect polymorphs, and the decrease of the particles specific surface area, which reduces the dissolving of Mn, makes the structure retain stability during lithium intercalation and deintercalation.
3.3 Electrochemical performance
3.3.1 Charge and discharge
Figure 3 shows that both charge/discharge profiles of lithium manganese oxide with or without F incorporation exhibit two platforms, demonstrating that the insertion and extraction of lithium ions occur in two stages [14] and the substitution of F does not change the spinel structure of the material. From charge-discharge curves, it is found that the cathode materials doped with F have initial discharge capacities of 105.9, 107.4, 113.2, 111.0 and 103.9 mA·h/g for z=0, 0.05, 0.10, 0.15 and 0.20, respectively, at 0.2C. It can be obviously seen that the initial discharge capacity of the Li1.03Co0.10Mn1.90FzO4-z cathode materials gradually increases first and then decreases with the increase of F content. This can explain that substitution of F- for O2- leads to an increase of Mn3+. As we know, the capacity of the lithium-manganese spinel LiMn2O4 depends on the amount of Mn3+. Therefore, a small amount of F-substitution will increase the discharge capacity. However, over 10% substitution of F- will cause the formation of MnF3 impurity. MnF3 diffraction is shown in XRD when z>10%. As an inactive substance, the formation of MnF3 results in the decrease of active substance content, so the discharge capacity of the cathode materials decreases. Thus, there is a lower discharge capacity than the sample with 10% F-substitution. Compared with the composition of Li1.03Co0.10Mn1.90F0.10O3.90, it is clear from Fig. 3 that the initial discharge capacity of Li1.03Co0.10Mn1.90F0.15O3.85 is 111.0 mA·h/g, which only reduces by 2.2 mA·h/g, while the sample Li1.03Co0.10Mn1.90F0.20O3.80 has a lower initial discharge capacity of 103.9 mA·h/g due to the fact that Li1.03Co0.10Mn1.90F3.85O0.15 has fine crystallinity and minor particle size which may has great benefit to migration and proliferation of Li+, and reduces the polarization of lithium manganate in the discharge process [15].
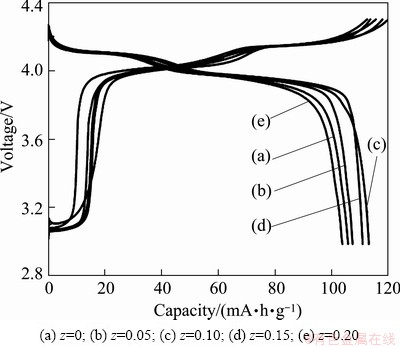
Fig. 3 Initial charge/discharge profiles of Li1.03Co0.10Mn1.90- FzO4-z
3.3.2 Cycling performance
The cycling performances of the samples with different amounts of F- substitution are shown in Fig. 4. It can be seen from the figure that the capacity retentions of Li1.03Co0.10Mn1.90FzO4-z with z=0.05 and 0.10 are 95.3% and 94.5%, respectively, which are slightly lower compared with Li1.03Co0.10Mn1.9O4 (97.8%). It is due to the fact that the amount of Mn3+ increasing results in the enhancement of Jahn-Teller effect and dissolution of Mn2+ which lead to the loss of the discharge capacity during cycling [15]. From the cycling results shown in Fig. 4, it can be seen that when z=0.20, the capacity retention reduces to 94.4%. However, for the sample with 15% of F-substitution, the capacity fading is only 3% after the 30th cycle, which is due to the fact that the increase of F- substitution amount maintains the stability of the structure of the spinel lithium manganese oxide in the charge and discharge process, improves the electrochemical stability and reduces the capacity attenuation of the spinel during cycle process. But from XRD, when z=15%, there is obvious MnF3 diffraction, which demonstrates that the amount of F substitution for O element reaches the maximum. With the increase of F amount, the content of MnF3 impurity will increase, so, it will result in the discharge capacity and cycling lifetime decrease. Combined cycle performance and discharge capacity, the Li1.03Co0.10Mn1.90F0.15O3.85 electrode shows excellent electrochemical performances.
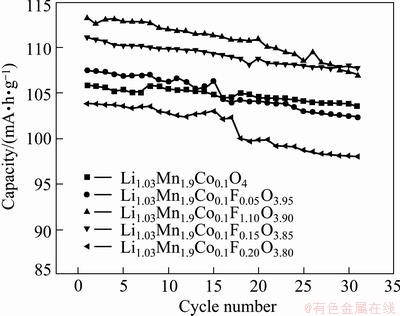
Fig. 4 Cycling performance of Li1.03Co0.10Mn1.90FzO4-z
4 Conclusions
Li1.03Co0.10Mn1.90FzO4-z (z=0, 0.05, 0.10, 0.15, 0.20) cathode materials were synthesized with the mixture of Li2CO3, LiF, Co2O3 and Mn2O3 by solid-state reaction. The XRD analysis reveals that the prepared Li1.03Co0.10Mn1.90FzO4-z materials have well-ordered spinel structure (space group Fd3m). The average particle size of F-substitution materials is greater than the un-doped Li1.03Co0.10Mn1.90O4 sample. The effects of different F-substitution content on Li1.03Co0.10Mn1.90O4 indicates that the prepared Li1.03Co0.10Mn1.90F0.15O3.85 sample exhibits a discharge capacity of 111.0 mA·h/g and a capacity retention of 97.0% after 30 cycles. Therefore, it can be concluded that electrochemical performance of spinel LiMn2O4 can be enhanced by combination of Li excess, substitution of Co and appropriate amount of F (15%).
References
[1] YU Fu-xin. Preparation and electrochemical properties of cathode materials for lithium-ion batteries [D]. Wuxi: Jiangnan University, 2008: 1-2. (in Chinese)
[2] GUO Hua-jun, XIANG Kai-xiong, CAO Xuan, LI Xin-hai, WANG Zhi-xing, LI Li-ming. Preparation and characteristics of Li2FeSiO4/C composite for cathode of lithium ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 166-169.
[3] HALIL S,  Effect of the Cr2O3 coating on electrochemical properties of spinel LiMn2O4 as a cathode material for lithium battery applications [J]. Solid State Ionics, 2010, 181: 1437-1444.
Effect of the Cr2O3 coating on electrochemical properties of spinel LiMn2O4 as a cathode material for lithium battery applications [J]. Solid State Ionics, 2010, 181: 1437-1444.
[4] GAO Y, DAHN J R. Correlation between the growth of the 3.3 V discharge plateau and capacity fading in Li1+xMn2-xO4 materials [J]. Solid State Ionics, 1996, 84(1-2): 33-40.
[5] XIAO Jin, ZHU Hua-li. Preparation and property of spinel LiMn2O4 material by Co-doping anti-electricity ions [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(2): 467-472.
[6] BUHRMESTER T, MARTIN M. X-ray absorption investigation on the ternary system lithium manganese oxide [J]. Solid State Ionics, 2000, 135(1-4): 267-272.
[7] AMDOUNI N, GENDRON F, MAUGER A, ZARROUK H, JULIEN C M. LiMn2-yCoyO4 (0≤y≤1) intercalation compounds synthesized from wet-chemical route [J]. Materials Science and Engineering A, 2006, 129(1-3): 64-75.
[8] BRUNO H F,  A A, NERILSO B, MARCOS F S T. Study of the potentiometric response of the doped spinel Li1.05Al0.02Mn1.98O4 for the optimization of a selective lithium ion sensor [J]. Electrochimica Acta, 2010, 55(20): 5659-5664.
A A, NERILSO B, MARCOS F S T. Study of the potentiometric response of the doped spinel Li1.05Al0.02Mn1.98O4 for the optimization of a selective lithium ion sensor [J]. Electrochimica Acta, 2010, 55(20): 5659-5664.
[9] THIRUNAKARAN R, KIM K T, KANG Y M, JAI Y L. Cr3+ modified LiMn2O4 spinel intercalation cathodes through oxalic acid assisted sol–gel method for lithium rechargeable batteries [J]. Materials Research Bulletin, 2005, 40(1): 177-186.
[10] LI Tao, QIU Wei-hua, ZHAO Hai-lei, LIU Jing-jing. Electrochemical properties of spinel LiMn2O4 and LiAl0.1Mnl.9O3.9F0.1 synthesized by solid-state reaction [J]. Journal of University of Science and Technology Beijing, 2008, 15(2): 187-191.
[11] LU Xing-he, TANG Zhi-yuan, ZHANG Na, HAN Dong, LI Hai-mei, NIU Lan-qin. Electrochemistry performance for the multiple doping spinel type Li1.02MxMn2-xQyO4-y [J]. Chinese Journal of Inorganic Chemistry, 2005, 21(9): 106-107.
[12] LIU Si-yin, LI Guo-fang, LI Guan-liang. Anion-cation synchronous substitution of LiAlxMn2-xOyFz by sol-gel-microwave method [J]. Chemistry World, 2008, 49(8): 453-456.
[13] DAI Yue-hua. Solid-state preparation and electrochemical properties of doped lithium manganese oxide [D]. Nanjing: Nanjing Normal University, 2007: 51-57. (in Chinese)
[14] SONG Gui-ming, ZHOU Yu, ZHOU Wen-yuan. A new synthesis technology of cathode material LiMn2O4 for lithium ion battery [J]. Journal of Inorganic Materials, 2001, 16(3): 486-490.
[15] PAN Lei, TIAN Jian-hua, SHAN Zhong-qiang, LIU Hao-jie, WU Feng. Study on the electrochemical performance of LiMn2O4 doped with Co3+ and F- [J]. Chinese Journal of Power Sources, 2007, 31(6): 450-452. (in Chinese).
掺F锂离子电池正极材料Li1.03Co0.10Mn1.90FzO4-z的制备及电化学性能
刘文静,张 云,王 辅,卢 超
四川大学 材料科学与工程学院,成都 610064
摘 要:以Li2CO3、Mn2O3、Co2O3及LiF为原料,采用高温固相法合成了掺F的Li1.03Co0.10Mn1.90FzO4-z锂电池正极材料。通过离子发射光谱(ICP)和电位分析法确定了材料的化学组成,用X-射线衍射(XRD)、扫描电子显微镜(SEM)和电化学测试仪分析了F掺杂量对材料结构、形貌和电池性能的影响。结果表明,掺F的Li1.03Co0.10Mn1.90FzO4-z正极材料为尖晶石结构,在F掺入量z≤0.10时,随着掺杂量的增加晶胞参数逐渐增加,当F掺杂量继续增加时,晶胞参数的增幅有所减小。适量的F-与金属离子Li+、Co+的复合掺杂提高了材料的放电比容量,同时增强了材料结构的稳定性。电化学性能测试表明,Li1.03Co0.10Mn1.90F0.15O3.85的首次放电比容量达到111.0 mA·h/g,0.2C倍率下30次循环后容量保持率为97.0%。
关键词:正极材料;固相反应;F掺杂;循环性能
(Edited by Xiang-qun LI)
Foundation item: Project (2011GZ0131) supported by the Sichuan Province Key Technology Support Program, China
Corresponding author: Yun ZHANG; Tel: +86-28-85410272; E-mail: y_zhang@scu.edu.cn
DOI: 10.1016/S1003-6326(13)62734-X