液相球磨法由氧化铋制备次碳酸铋的动力学
来源期刊:中国有色金属学报(英文版)2014年第9期
论文作者:叶龙刚 蒋 叶 唐朝波 陈永明 唐谟堂
文章页码:3001 - 3007
Key words:bismuth oxide; ball-milling transformation; bismuth subcarbonate; kinetics; shrinking core model
摘 要:为解决传统次碳酸铋生产过程中成本高和环境污染问题,提出采用液相球磨法由氧化铋制备次碳酸铋的新工艺,研究用碳酸氢氨和氧化铋制备次碳酸铋反应过程的动力学,考察反应温度、氧化铋粒度、液固比以及碳酸氢氨浓度对氧化铋转化率的影响。结果表明,在9~30 °C的范围内,升高反应温度、减小氧化铋粉末的粒度、扩大液固比以及提高碳酸氢氨浓度均有利于氧化铋转化率的提高。对反应产物的表征分析表明,产品的纯度较高、杂质少;SEM结果显示产品次碳酸铋主要呈针棒状形态。反应过程受产物层的扩散控制,可用未反应收缩核模型描述,反应的表观活化能为9.783 kJ/mol,同时获得了描述反应过程的半经验动力学方程。
Abstract: In order to solve the problems of environment pollution and high cost in traditional process of bismuth subcarbonate preparation, a new process using ball-milling transformation method from NH4HCO3 and Bi2O3 was proposed. Additionally, the kinetics of bismuth subcarbonate preparation was studied. Effects of reaction temperature, particle size of bismuth oxide, solid-to-liquid ratio and concentrations of ammonium bicarbonate on the conversion rate of bismuth oxide were studied. The results indicate that the conversion rate of bismuth oxide significantly increased under the conditions of higher temperature, smaller particle size, higher concentration of ammonium bicarbonate and smaller solid-to-liquid ratio. The XRD and ICP-AES analyses show that the purity of product is high. The reaction kinetics with activation energy of 9.783 kJ/mol was analyzed by shrinking core model, and the whole transformation process is controlled by solid product layer diffusion. A semi-empirical kinetics equation was obtained to describe the conversion process.
Trans. Nonferrous Met. Soc. China 24(2014) 3001-3007
Long-gang YE, Ye JIANG, Chao-bo TANG, Yong-ming CHEN, Mo-tang TANG
School of Metallurgy and Environmental, Central South University, Changsha 410083, China
Received 20 August 2013; accepted 23 December 2013
Abstract: In order to solve the problems of environment pollution and high cost in traditional process of bismuth subcarbonate preparation, a new process using ball-milling transformation method from NH4HCO3 and Bi2O3 was proposed. Additionally, the kinetics of bismuth subcarbonate preparation was studied. Effects of reaction temperature, particle size of bismuth oxide, solid-to-liquid ratio and concentrations of ammonium bicarbonate on the conversion rate of bismuth oxide were studied. The results indicate that the conversion rate of bismuth oxide significantly increased under the conditions of higher temperature, smaller particle size, higher concentration of ammonium bicarbonate and smaller solid-to-liquid ratio. The XRD and ICP-AES analyses show that the purity of product is high. The reaction kinetics with activation energy of 9.783 kJ/mol was analyzed by shrinking core model, and the whole transformation process is controlled by solid product layer diffusion. A semi-empirical kinetics equation was obtained to describe the conversion process.
Key words: bismuth oxide; ball-milling transformation; bismuth subcarbonate; kinetics; shrinking core model
1 Introduction
Bismuth is a green and non-toxic metal. Since 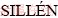 , a Swedish scientists, discovered the Sillén phase compound with layered structure by (Bi2O2)2+ layers and the layers of X (X involving halogen, carbonate and other ionic groups) arranged alternately for the first time in 1942, bismuth subnitrate and bismuth subcarbonate as the deep-processing products of bismuth, which are of all Sillén phase compounds [1,2], have been widely applied in medical field, analytical reagents and bismuth compounds preparation [3-6]. The commercial route for the production of bismuth subcarbonate used bismuth nitrate as raw material, which was prepared by bismuth and nitric acid, and then bismuth compound was synthesized by the hydrolytic reactions of bismuth nitrate. A large amount of toxic gases and nitrogen dioxide were generated in this process, and it also has the shortcomings of consuming mass of acid and alkali reagents, having a long process and environmental pollution. CHEN et al [7] prepared bismuth subcarbonate nanoparticles by water-in-oil microemulsion-assisted hydrothermal process. DONG et al [8] studied rose-like monodisperse bismuth subcarbonate synthesized by one-pot template-free method. CHENG et al [9] investigated bismuth subcarbonate nanomaterials synthesized by shape-controlled. CAO et al [10] used hydrothermal preparation means to prepare (BiO)2CO3 with persimmon-like microstructure. These processes all have a long process as well as complicated device and high cost.
, a Swedish scientists, discovered the Sillén phase compound with layered structure by (Bi2O2)2+ layers and the layers of X (X involving halogen, carbonate and other ionic groups) arranged alternately for the first time in 1942, bismuth subnitrate and bismuth subcarbonate as the deep-processing products of bismuth, which are of all Sillén phase compounds [1,2], have been widely applied in medical field, analytical reagents and bismuth compounds preparation [3-6]. The commercial route for the production of bismuth subcarbonate used bismuth nitrate as raw material, which was prepared by bismuth and nitric acid, and then bismuth compound was synthesized by the hydrolytic reactions of bismuth nitrate. A large amount of toxic gases and nitrogen dioxide were generated in this process, and it also has the shortcomings of consuming mass of acid and alkali reagents, having a long process and environmental pollution. CHEN et al [7] prepared bismuth subcarbonate nanoparticles by water-in-oil microemulsion-assisted hydrothermal process. DONG et al [8] studied rose-like monodisperse bismuth subcarbonate synthesized by one-pot template-free method. CHENG et al [9] investigated bismuth subcarbonate nanomaterials synthesized by shape-controlled. CAO et al [10] used hydrothermal preparation means to prepare (BiO)2CO3 with persimmon-like microstructure. These processes all have a long process as well as complicated device and high cost.
XIA et al [11] prepared α-Bi2O3 through low-temperature oxidation process, and the results demonstrated that bismuth oxide was stable. The reaction conversion rate of bismuth oxide and ammonium bicarbonate solution was low in the natural state. Mechanical activation can accelerate the chemical reaction [12,13] due to the grain refinement and the lattice energy and defects increasing, so it has been widely used in materials preparing [14,15] and metallurgical process enhancing [16]. Zirconia ball is known to be a strong active medium in the milling process by surface friction and impact on the material, and it has been a intensified method for increasing the reaction conversion [17,18].
In this work, a new process using bismuth oxide and ammonium bicarbonate as raw materials to prepare bismuth subcarbonate was proposed by liquid ball-milling transformation method. The new process could avoid to yielding NOx toxic gases which was an inevitable by-product in the traditional process, and the mother solution from ball-milling process can be recycled after being treated, so it is a environment-friendly process. In the present work, the effects of temperature, particle size of Bi2O3, solid-to-liquid ratio and concentration of ammonium bicarbonate on the conversion rate of Bi2O3 were interpreted, and reaction kinetics was analyzed.
2 Experimental
2.1 Materials
The bismuth oxide used in this experiment was provided by Jingtang Bismuth Company, Hunan Province, China. The chemical composition is listed in Table 1. The sample is a high-purity product and the content of Bi2O3 is 99.951%. NH4NCO3 used for reacting with Bi2O3 is analytical grade.
Table 1 Chemical composition of bismuth oxide (mass fraction, %)

2.2 Methods
The conversion experiments were carried out in a 1000 mL spherical glass reactor equipped with a mechanical stirrer and a temperature control unit, and activation medium was zirconia ball of 1 cm in diameter. 200 mL of solution containing specific NH4HCO3 was charged into the reactor. When the desired stirring speed and reaction temperature achieved their set values, the solid Bi2O3 was added into the reactor, and this moment was defined as the experiment start time. After the predetermined experimental time, the sample solution was taken out to filter and dry at 60 °C for 48 h.
A certain amount (m1) of dried product was charged in an alumina crucible. After the total mass was measured, the crucible with sample was settled in the hot zone of the electric furnace to calcine the sample at 500 °C for 2 h. Yellow bismuth oxide powder was got after being calcined, and it was cooled to room temperature in a desiccator and then it was weighed (m2). The conversion rate of bismuth oxide was calculated based on the mass difference before and after calcination. Related possible reactions were as follows:
Bi2O3+NH4HCO3=(BiO)2CO3+NH3+H2O (1)
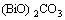 =
=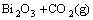 (2)
(2)
Bi2O3 and HCO3- could react sufficiently under ball-milling [19], and products were (BiO)2CO3 and OH-. The conversion rate of Bi2O3 was calculated by the following equation:
 (3)
(3)
where R is the conversion rate of Bi2O3; m1 and m2 are the masses of sample before and after calcined respectively.
3 Results and discussion
3.1 Effect of operation parameters on conversion rate of Bi2O3
The factors that might influence the conversion rate of Bi2O3 including ammonium bicarbonate concentration, the ratio of solid-to-liquid, reaction temperature and particle size of Bi2O3 were studied.
3.1.1 Effect of reaction temperature
The effect of temperature on conversion was investigated in the range from 282 to 323 K, at NH4HCO3 concentration 2 mol/L, particle size of Bi2O3 0.061-0.074 mm and solid-to-liquid 1:50. The relationship between conversion rate of Bi2O3 and reaction temperature is shown in Fig. 1(a).
As shown in Fig. 1(a), the conversion rate increases obviously with time and temperature increasing. The final conversion rate of Bi2O3 increases from 87.08% to 93.38% when the temperature changes from 282 to 303 K. Nevertheless, the conversion rate drops with temperature growing when the reaction temperature is over 303 K. The final conversion rate downs to 73.38% at 323 K. It can be explained that the reaction kinetics was accelerated by high temperature, but at the meanwhile, ammonium bicarbonate was decomposed to ammonia due to the high temperature [20,21], so actual concentrate of ammonium bicarbonate was reduced.
3.1.2 Effect of particle size of Bi2O3
The experiments were conducted in the particle size range of 0.026-0.038 mm to 0.250-0.180 mm, maintaining the ammonium bicarbonate concentrate at 2 mol/L, temperature at 293 K, and solid-to-liquid ratio at 1:50. The relationship between conversion rate of Bi2O3 and particle size of Bi2O3 is shown in Fig. 1(b).
As can be seen from Fig. 1(b), the conversion rate of Bi2O3 increases as the particle size of Bi2O3 decreases. When the particle size decreases from 0.250-0.180 mm to 0.026-0.038 mm, the final conversion rate correspondingly increases from 81.85% to 93.46%. Reducing the size of Bi2O3 particle is equivalent to increase the reaction surface. The gap is not obvious due to the little difference of particle size, but a small particle size has a positive effect on conversion rate.
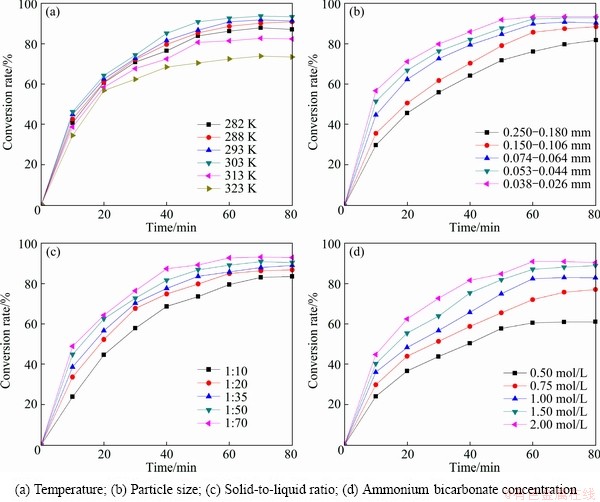
Fig. 1 Effects of operation parameters on conversion rate
3.1.3 Effect of solid-to-liquid ratio
The effect of solid-to-liquid ratio was investigated by running experiments using the solid-to-liquid ratio in the range from 1:10 to 1:70, when the temperature was 293 K, concentration of particle size of Bi2O3 was 0.061-0.074 mm and ammonium bicarbonate was 2 mol/L. The relationship between conversion rate of Bi2O3 and solid-to-liquid ratio is shown in Fig. 1(c).
When the solid-to-liquid ratio increases from 1:10 to 1:70, the final conversion rate of Bi2O3 increases from 83.45% to 92.86%. This indicates that increasing solid- to-liquid ratio could enhance the conversion rate of Bi2O3. There is no significant change on the conversion of Bi2O3 with solid-to-liquid ratio increasing further. This can be explained that the ammonium bicarbonate is excess compared with calculated amount for the conversion of Bi2O3.
3.1.4 Effect of ammonium bicarbonate concentration
The object of the experiments is to observe the influence of ammonium bicarbonate concentration on the conversion rate of Bi2O3. These experiments were carried out at 293 K with solid-to-liquid ratio of 1:50 and particle size of Bi2O3 of 0.061-0.074 mm. The concentration of ammonium bicarbonate was charged from 0.5 mol/L to 2 mol/L. The experimental results are shown in Fig. 1(d).
As shown in Fig. 1(d), the conversion rate of Bi2O3 increases quickly with the concentration of ammonium bicarbonate increasing. For concentration of NH4HCO3 increasing from 0.5 mol/L to 2 mol/L, the final conversion rate increases from 61.04% to 90.37%. This illustrates that the concentration of ammonium bicarbonate has a remarkable effect on conversion rate of Bi2O3, because according to the mass action law, increasing the concentration of reactants could accelerate the reaction rate.
3.2 Characterization of product
The bismuth subcarbonate was obtained under the following conditions: solid-to-liquid ratio 1: 50, particle size of Bi2O3 0.061-0.074 mm, concentration of ammonium bicarbonate 2 mol/L and temperature 293 K. XRD, SEM and ICP-AES analyses of the product are presented in Fig. 2, Fig. 3 and Table 2, respectively.
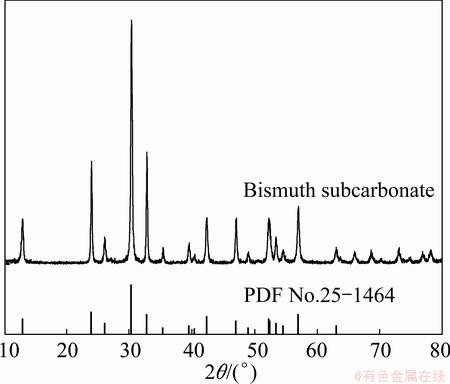
Fig. 2 XRD patterns of bismuth subcarbonate
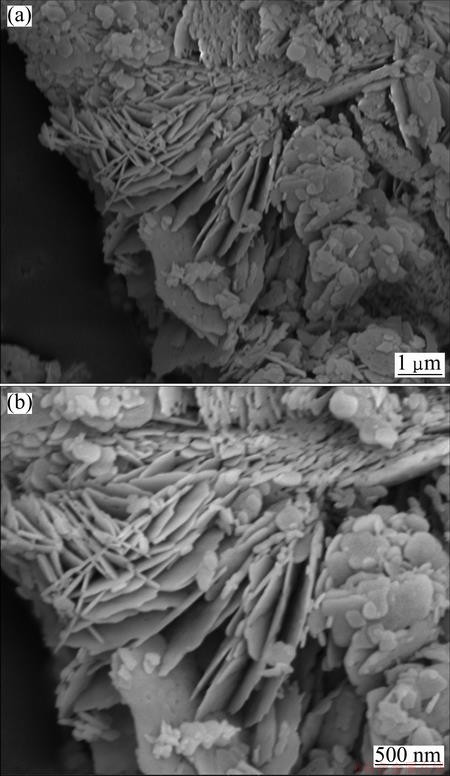
Fig. 3 SEM images of bismuth subcarbonate at different magnifications
Comparing the XRD pattern with the JCPDS files, it illustrates that almost all the peaks of product are identified as bismuth subcarbonate (JCPDS card, No.25-1464), and the impurity can be identified as bismuth oxide (JCPDS card, No.65-2366). SEM images of product illustrate that the morphology is nearly flaky.
As shown in Table 2, the contents of all impurities involving S, Sb, Sn, P, Fe, Ca, Na and Mg are lower than 0.02%, and the total amount of impurity is below 0.1%. This illustrates that the product has a high purity and less impurity enters the product in preparation process.
Table 2 Chemical composition of bismuth subcarbonate (mass fraction, %)
3.3 Kinetic analysis
The reaction process of Bi2O3 could be explained by a shrinking core model [22]. If the reaction rate is controlled by diffusion through a product layer, the kinetics equation is as follows [23,24]:
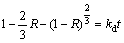 (4)
(4)
If the reaction is controlled by a surface reaction, the kinetics equation is as follows [25]:
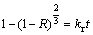 (5)
(5)
where R is the reacted fraction; t is the reaction time; kd and kr are the rate constants, respectively.
Equation (4) or (5) reveals that if the diffusion through the product layer or the surface reaction controls reactions rate, there must be a linear relationship between the 1-(2/3)R-(1-R)2/3 or 1-(1-R)2/3 and time. The slope is the apparent rate constant kd or kr.
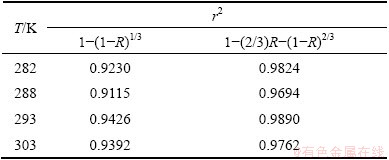
The results of the analysis including the correlation coefficient (r2) are shown in Table 3. As can be seen from Table 3, the diffusion controlled model fits better in two models. Using the apparent rate constant (kd) obtained by Eq. (4), the Arrhenius plot is obtained (Fig. 4(a)). The activation energy is calculated to be 9.8138 kJ/mol. This value clearly confirms that this process was controlled by the diffusion through a product layer [26].
Table 3 Correlation coefficient (r2) of two kinetics models at different temperatures
A series of well-fitted plots of 1-(2/3)R-(1-R)2/3 versus time at different temperatures, particle sizes, solid-to-liquid ratios, ammonium bicarbonate concentrations are given in Fig. 5.
In order to determine the effect of temperature, particle size, solid-to-liquid ratio (ρ), concentration of ammonium bicarbonate on the reaction kinetics, the following semi-empirical model was established [27]:
1-(2/3)R-(1-R)2/3=k0CNH4HCO3aDbρcexp[-E/(RT)]t (6)
where T is the temperature; CNH4HCO3 is the concentration of ammonium bicarbonate; D is the particle size; k0 is the apparent reaction rate coefficient; and E is the activation energy.
To different ammonium bicarbonate concentrations, when other parameters maintain constant, Eq. (6) could be written as
1-(2/3)R-(1-R)2/3=K1CNH4HCO3at (7)
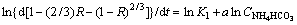 (8)
(8)
where  is the slope of the straight lines corresponding to different ammonium bicarbonate concentrations in Fig. 4(d). The values of
is the slope of the straight lines corresponding to different ammonium bicarbonate concentrations in Fig. 4(d). The values of 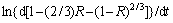 versus ln CNH4HCO3are plotted to from a straight line, and from the slop, it is calculated a=0.82683. In a similar way the empirical reaction orders obtained for particle size and solid-to-liquid ratio are -0.36567 and 0.24977, respectively, as shown in Fig. 5.
versus ln CNH4HCO3are plotted to from a straight line, and from the slop, it is calculated a=0.82683. In a similar way the empirical reaction orders obtained for particle size and solid-to-liquid ratio are -0.36567 and 0.24977, respectively, as shown in Fig. 5.
Substituting the values of a, b, c and E into Eq. (6), the value of k0 is calculated to be about 0.0018274 when using the equation to fit different straight lines in Fig. 4. So, the kinetics equation was established as follows: 1-(2/3)R-(1-R)2/3=0.0018274CNH4HCO30.82683D-0.36567·ρ0.24977exp[-9.8138/(RT)]t.
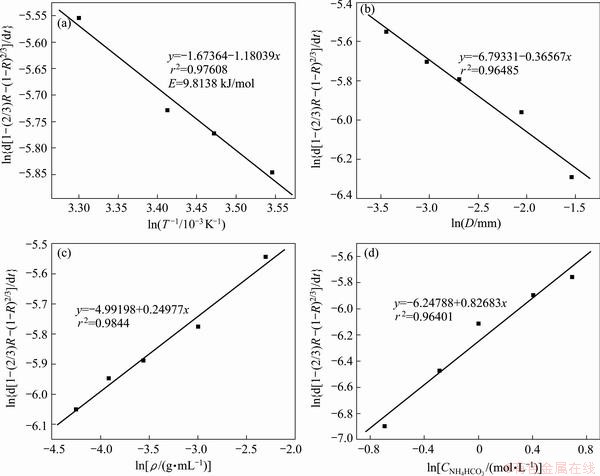
Fig. 4 Plots of ln{d[1-(2/3)R-(1-R)2/3]/dt} versus 1/T, ln ρ, ln D, ln CNH4HCO3
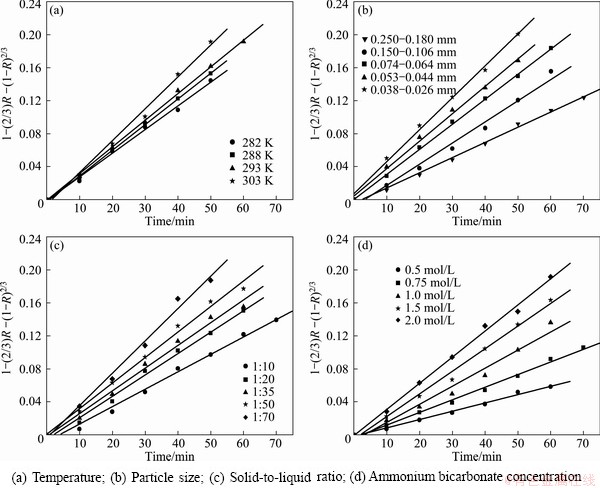
Fig. 5 Plots of t versus 1-(2/3)R-(1-R)2/3 at different operation parameters
4 Conclusions
1) The results show that temperature, particle size, solid-to-liquid ratio and concentration of ammonium bicarbonate have favorable influence on the conversion rate of Bi2O3. Small particle size and solid-to-liquid ratio, large concentration of ammonium and high temperature below 303 K will increase the conversion rate.
2) The conversion rate of Bi2O3 is controlled by the diffusion through the ash layer around the shrinking unreacted core and the activation energy is calculated to be 9.8138 kJ/mol.
3) The reaction kinetics could be described by the following equation:
1-(2/3)R-(1-R)2/3=0.0018274CNH4HCO30.82683D-0.36567·ρ0.24977exp[-9.8138/(RT)]t.
References
[1] YANG Nan, SUN Hong-zhe. Biocoordination chemistry of bismuth: Recent advances [J]. Coordination Chemistry Review, 2007, 251(17-20): 2354-2366.
[2] KOZAR R W, WALDMANN T A, ATCHER R W, GANSOW O A. Radionuclide-conjugated monoclonal antibodies: A synthesis of immunology, inorganic chemistry and nuclear science [J]. Trends in Biotechnology, 1986, 4(10): 259-264.
[3] ZHOU Yu, WANG Wen, JIA De-chang, YE Feng. Synthesis of SrBi2Ta2O9 nanocrystalline powder by a modified sol-gel process using bismuth subnitrate as bismuth source [J]. Materials Chemistry and Physics, 2002, 37(15): 60-64.
[4] DAVID H A, JOSHUA N A, PATRICK D H, NATHANIEL J S, RAM S M. Bismuth compounds in organic synthesis. Bismuth nitrate catalyzed chemoselective synthesis of acylals from aromatic aldehydes [J]. Tetrahedron, 2004, 60(16): 3675-3679.
[5] ALI R P, FATEMEH F. Selective nitration of phenols using bismuth subnitrate/charcoal in the presence of trichloroisocyanuric acid under aprotic conditions [J]. Chinese Chemical Letters, 2010, 21(11): 1283-1286.
[6] REDDY Y T, RAJITHA B, REDDY P N, KUMAR B S, RAO V P. Bismuth subnitrate catalyzed efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones: An improved protocol for the Biginelli reaction [J]. Synthetic Communications, 2004, 34(20): 3821-3825.
[7] CHEN Rong, CHENG Gang, MAN Ho-So, WU Jiang, CHE Chi-ming, SUN Hong-zhe. Bismuth subcarbonate nanoparticles fabricated by water-in-oil microemulsion-assisted hydrothermal process exhibit anti-Helicobacter pylori properties [J]. Materials Research Bulletin, 2010, 45(5): 654-658.
[8] DONG F, LEE S C, WU Z B, HUANG Y, FU M, HO W K, ZOU S C, WANG B. Rose-like monodisperse bismuth subcarbonate hierarchical hollow microspheres: One-pot template-free fabrication and excellent visible light photocatalytic activity and photochemical stability for NO removal in indoor air [J]. Hazardous Materials, 2011, 195(15): 346-354.
[9] CHENG Gang, YANG Han-min, RONG Kai-feng, LU Zhong, YU Xiang-lin, CHEN Rong. Shape-controlled solvothermal synthesis of bismuth subcarbonate nanomaterials [J]. Solid State Chemistry, 2010, 183(8): 1878-1883.
[10] CAO Xiao-feng, ZHANG Lei, CHEN Xue-tai, XUE Zi-ling. Persimmon-like (BiO)2CO3 microstructures: Hydrothermal preparation, photocatalytic properties and their conversion into Bi2S3 [J]. Cryst Eng Comm, 2011, 13(6): 1939-1945.
[11] XIA Ji-yong, TANG Mo-tang, CHEN Cui, JIN Shen-ming, CHEN Yong-ming. Preparation of α-Bi2O3 from bismuth powders through low-temperature oxidation [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2289-2294.
[12] MARTIN K B, HAUKE C S. Mechanochemistry: The mechanical activation of covalent bonds [J]. Chemical Review, 2005, 105(8): 2921-2948.
[13] BOLDYREV V V. Mechanochemistry and mechanical activation of solids [J]. Russian Chemical Reviews, 2006, 75(3): 177-189.
[14] KIM J K, CHERUVALLY G, CHOI J W, KIM J U, AHN J H, CHO G B, KIM K W, AHN H J. Effect of mechanical activation process parameters on the properties of LiFePO4 cathode material [J]. Journal of Power Sources, 2007, 166(1): 211-218.
[15] MARKMAITREE T, REN R M, SHAW L. Enhancement of lithium amide to lithium imide transition via mechanical activation [J]. The Journal of Physical Chemistry B, 2006, 110(41): 20710-20718.
[16] MAURICE D, HAWK J A. Ferric chloride leaching of mechanically activated chalcopyrite [J]. Hydrometallurgy, 1998, 49(1-2): 103-123.
[17] LI Liang, YANG You-wen, LI Guang-hai. Conversion of a Bi nanowire array to an array of Bi–Bi2O3 core–shell nanowires and Bi2O3 nanotubes [J]. Small, 2006, 2(4): 548-553.
[18] BOLDYREV V V. Mechanochemistry and mechanical activation of solids [J]. Russian Chemical Reviews, 2006, 75(3): 537-543.
[19] YU Zheng-guang, YANG Bang-chao, LU Yun. Synthesis of spherical bismuth oxide powder [J]. The Chinese Journal of Process Engineering, 2003, 3(3): 261-264.
[20] WANG Shu-juan, LIU Fang, CHEN Chang-he, XU Xu-chang. Life cycle emissions of greenhouse gas for ammonia scrubbing technology [J]. Korean Journal of Chemical Engineering, 2007, 24(3): 495-498.
[21] JAMES T Y, KEVIN P R, HENRY W P. Regenerable aqua ammonia process for CO2 sequestration [J]. Division of Fuel Chemistry, 2004, 49(1): 247-248.
[22] LUO Wei, FENG Qi-min, OU Le-ming, ZHANG Guo-fan, CHEN Yun. Kinetics of saprolitic laterite leaching by sulphuric acid at atmospheric pressure [J]. Minerals Engineering, 2010, 23(6): 458-462.
[23] QIU Ting-sheng, NIE Gang-hua, WANG Jun-feng, CUI Li-feng. Kinetics process of oxidative leaching of chalcopyrite under low oxygen pressure and low temperature [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(2): 418-422.
[24] BIAN Xue, YIN Shao-hua, LUO Yao, WU Wen-yuan. Leaching kinetics of bastnaesite concentrate in HCl solution [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 2306-2310.
[25] XIE Ke-qiang, YANG Xian-wan, WANG Ji-kun, YAN Jiang-feng, SHEN Qing-feng. Kinetic study on pressure leaching of high iron sphalerite concentrate [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(1): 187-194.
[26] AYDOGAN S, UCAR G, CANBAZOGLU M. Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution [J]. Hydrometallurgy, 2006, 81(1): 45-51.
[27] LIU Wei, TANG Mo-tang, TANG Chao-bo, HE Jing, YANG Sheng-hai, YANG Jian-guang. Dissolution kinetics of low grade complex copper ore in ammonia-ammonium chloride solution [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 910-197.
叶龙刚,蒋 叶,唐朝波,陈永明,唐谟堂
中南大学 冶金与环境学院,长沙 4100083
摘 要:为解决传统次碳酸铋生产过程中成本高和环境污染问题,提出采用液相球磨法由氧化铋制备次碳酸铋的新工艺,研究用碳酸氢氨和氧化铋制备次碳酸铋反应过程的动力学,考察反应温度、氧化铋粒度、液固比以及碳酸氢氨浓度对氧化铋转化率的影响。结果表明,在9~30 °C的范围内,升高反应温度、减小氧化铋粉末的粒度、扩大液固比以及提高碳酸氢氨浓度均有利于氧化铋转化率的提高。对反应产物的表征分析表明,产品的纯度较高、杂质少;SEM结果显示产品次碳酸铋主要呈针棒状形态。反应过程受产物层的扩散控制,可用未反应收缩核模型描述,反应的表观活化能为9.783 kJ/mol,同时获得了描述反应过程的半经验动力学方程。
关键词:氧化铋;球磨转化;次碳酸铋;动力学;收缩核模型
(Edited by Xiang-qun LI)
Foundation item: Project (50774099) supported by the National Natural Science Foundation of China
Corresponding author: Chao-bo TANG; Tel: +86-731-88830470; E-mail: tangchaobo9043@163.com
DOI: 10.1016/S1003-6326(14)63437-3

