
Synthesis and electrochemical performance of a novel nano sized Sn2SbNi composite
GUO Hong(郭 洪)1, 2, LIN Xue-fei(林雪飞) 2, CHEN Yi-san(陈益山) 2
1. School of Chemical Science and Technology, Yunnan University, Kunming 650091, China;
2. School of Chemistry and Chemical Engineering, Qujing Normal University, Qujing 655000, China
Received 6 July 2009; accepted 30 December 2009
_____________________________________________________________________________________________________
Abstract: Chemical reduction method was employed to prepare nano-sized Sn2SbNi alloy composites used as anode material for rechargeable lithium ion batteries. This strategy was adopted to combine the virtues of both active/inactive and active/active alloys to fabricate a Sn2SbNi alloy powder with two active components and one inactive component. The two active components can realize the high capacity feature of electrode and can make the volume change of electrode take place in a stepwise manner due to the different lithiation potentials of two active components, leading to a stable cycling performance. Sn2SbNi alloy provides a reversible specific capacity over 640 mA?h/g with an excellent cyclic ability. The Sn-Sb-Ni alloy composite material shows to be a good candidate anode material for the lithium ion batteries.
Key words: Li-ion batteries; Sn2SbNi composite; anode materials; chemical reduction method
_____________________________________________________________________________________________________
1 Introduction
Rechargeable lithium-ion batteries with their light weight and high energy density have become an important source for powering in many applications. Although the widely current used Li-ion battery is a successful technology in conquering the battery market, the new challenges to achieve higher energy density, higher rate, higher stability, longer cycle life, and improved safety stimulate the research for grand new breakthroughs in electrode materials[1-2]. Among the varieties of anode materials, Sn-based materials with high theoretical specific capacity (994 mA?h/g) have been projected as one of the most promising candidates in substitute of the already-commercialized graphite, due to their high capacity, high packing density and safe thermodynamic potentials compared with carbonaceous materials for lithium secondary batteries. However, they usually undergo severe structural and volume changes during the process of Li uptake and removal, which results in mechanical disintegration of the electrode and consequent capacity fade, and greatly limits the commercialization. Many efforts, therefore, have been devoted to improve the cycling duration of Sn-based system[3-5], which includes using nano sized intermetallics or composite host material instead of pure metal. Related reports have revealed that reducing the size of active particles is an effective method to improve the cycleability of the alloy materials for the case of Sn, such as active/active SnSb, and active/inactive SnCu and SnNi[6-9].
In the present work, a strategy was adopted to combine the virtues of both active/inactive and active/active alloys to fabricate a Sn2SbNi alloy powder with two active components and one inactive component. The nano-sized grains can homogenize the expansion/contraction strains, and thus relieve the mechanical stress and buffer the volume change of the particles caused by the lithiation and delithiation processes. The two active components can realize the high capacity feature of electrode and can make the volume change of electrode take place in a stepwise manner due to the different lithiation potentials of two active components, leading to a stable cycling performance. Though Ni shows to be inert with Li, its excellent flexibility and electric conductive character will contribute greatly to the cycling stability of electrode. Therefore, Sn2SbNi alloy is expected to have high specific capacity and good cycling stability. The effect of chemical composition on the electrochemical properties of Sn2SbNi electrode was investigated in terms of cyclic performance and cyclic voltammetry (CV) features.
2 Experimental
Aqueous solutions were used for the synthesis of SnxSbNi powder, namely, solution A formed by SnCl2?2H2O (99.9%, STREM Chemicals), SbCl?H2O (99.9%, STREM Chemicals), and complexant citrates; and solution B formed by NaOH and NaBH4(99.9%, STREM Chemicals). The procedure follows basically the procedure recommended in Refs.[10-11]. The precipitate was filtrated and subsequently washed with distilled water, 0.35 mol/L HCl and acetone, followed by drying at 120 ℃ in vacuum. X-ray diffraction (XRD) was carried out to identify the phase composition of synthesized powders over the 2θ range from 10? to 90? using a Rigaku D/max-A diffractometer with Cu Kα radiation. The particle morphologies and phase compositions of the synthesized SnxSbNi powders were characterized by scanning electron microscope (SEM), transmission electron microscope (TEM, JEM-100CXⅡ); and the corresponding lattice structure was identified by selected area electron diffraction (SAED) technique. For electrochemical performance evaluation, half-cell studies were performed. In the experimental SnxSbNi electrode, C (acetylene black) powder and polyvinylidene fluoride (PVDF) were used as conductive additive and binder, respectively. The synthesized SnxSbNi powders were mixed with acetylene black and PVDF dissolved in N-methyl-pyrrolidinone in the mass ratio of 85?10?5 to form slurry, which was painted on a copper foil used as current collector. After solvent evaporation, the electrode was pressed and dried at 120 ℃ under vacuum for 48 h.
The cells were assembled in argon filled glove-box. Metallic lithium foil was used as counter electrode. The electrolyte was 1 mol/L LiPF6 (Merck, battery grade) in a mixture of ethyl carbonate (EC) and dimethyl carbonate (DMC) (1?1 in volume ratio). Celgard 2400 polyethylene was used as the separator. Cycling tests were carried out at the charge and discharge conditions of 100 mA/g in the voltage range of 0.01-1.5 V versus Li/Li+ by LAND BT-10 tester (Wuhan, China). Cyclic voltammetry was performed between 0.01 and 1.5 V with scan rate of 0.05 mV/s.
3 Results and discussion
XRD pattern of the synthesized Sn2SbNi alloy composite powder is shown in Fig.1. It reveals that all the products are multiphase compounds, which mainly consist of SnSb, and Sn, Ni metals. No peaks assignable to raw materials were identified, indicating that all oxides have been reduced completely. No obvious peaks corresponding to metal Sb were found in XRD pattern, implying that most of the Sb component has combined with Sn to form SnSb intermetallic, and some other Sb may exist in amorphous state, as evidenced by the relatively high background of XRD pattern.
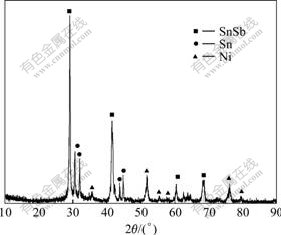
Fig.1 XRD pattern of Sn2SbNi alloy composite powders
The SEM image of synthesized alloy composite powders is presented in Fig.2. The synthesized powder showed mainly spherical conglomeration from 40 to 100 nm. EDS analysis of the synthesized Sn2SbNi alloy composite powders, shown in Fig.3, gave a Sn to Sb to Ni molar ratio of 52.77?24.51?22.72, showing seemingly that tin-rich composite is obtained. The chemical composition of synthesized material is closed to the starting molar ratio of Sn to Sb to Ni, i.e. 2?1?1 before reaction.
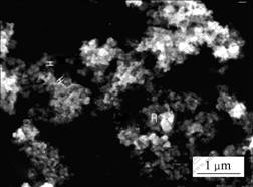
Fig.2 SEM image of Sn2SbNi composite powders
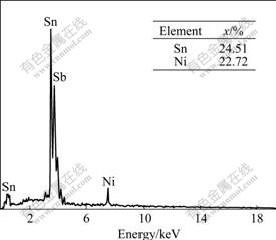
Fig.3 EDS spectrum of Sn2SbNi powder
Fig.4 shows TEM image of a Sn2SbNi alloy powder prepared by chemical reduction. It shows clear grain shapes, and the crystal edges can be observed. The granularity of particles is 10-30 nm, illumining that the relatively large particles observed in SEM are configuration from little particles. A multi-crystal structure with clear crystal loops of diffraction structure can be detected by SAED, as illustrated in Fig.4. This also indicates again that the single spherical particles observed in SEM are practically aggregates of nano-sized grains.

Fig.4 TEM image of Sn2SbNi alloy powders(The inset is the selected area electron diffraction of a area of Sn2SbNi alloy particle, showing multi-crystalline characteristic of SnSb)
Lithium ions inserting into and extracting from SnxSbNi alloy composite electrode are defined as discharge and charge processes, respectively. The cyclic performance profiles of SnxSbNi alloy composite electrode (x=1, 2 and 3) at 100 mA/g are shown in Fig.5. The first discharge capacity of SnSbNi, Sn2SbNi, and Sn3SbNi electrode are 894, 961 and 1 020 mA?h/g, respectively. The increase in initial discharge capacity with the increase of Sn content is mainly attributable to the high lithium storage capacity of Sn. In the following 20 cycles, except Sn3SbNi, the synthesized SnSbNi and Sn2SbNi alloy electrodes exhibit good stability with discharge capacities of about 500 mA?h/g and about 710 mA?h/g, and the columbic efficiencies of 97.8% and 97.5%, respectively. The results are understandable because of the nano-structural and the multi-element characteristics. The former can accommodate the volume change caused by the lithiation/delithiation of electrode, while the latter allows the lithiation/delithiation of SnxSbNi electrode to take place at different potentials, i.e. in a stepwise behavior, thus makes the volume change of electrode occur more smoothly. These will eventually result in a good structural and cyclic stability of electrode. The inactive matrix Ni can buffer the volume change of Sn and Sb in the electrochemical cycling process, and can also prevent the aggregation of the alloy particles in a certain extent, thus will also contribute to the good cycleability of electrode. In addition, the good electronic conductivity of component nickel should also be responsible for the better electrochemistry performance of electrode. However, when the molar ratio of Sn to Sb to Ni arrived at 3?1?1, the capacity of Sn3SbNi electrode faded drastically from 1 020 to 383 mA?h/g after 20 cycles. The relatively excessive amount of Sn in SnxSbNi alloy electrode will result in large volume expansion upon lithiation of electrode, which is considered to be the main reason of the poor cycling behavior of electrode. The maximal volume expansion of tin can reach as high as 300% according to Ref.[12]. Another reason is that more Sn content in SnxSbNi composite alloy may easily congregate together upon electrochemical cycling to form large Sn particles, which will readily result in mechanical pulverization of the electrode and consequent capacity drop of electrode. The increased kinetic polarization of tin-rich electrode is another reason for the unstable cyclic ability.
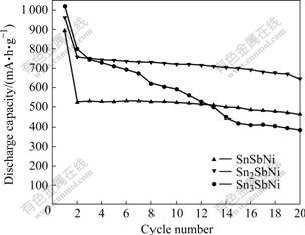
Fig.5 Cyclic performance of SnxSbNi alloy composite electrodes at 100 mA/g (x=1, 2 and 3)
The initial capacity loss is about 200 mA?h/g for synthesized Sn2SbNi, which is similar to that of most reported alloy anode electrodes fabricated by liquid coprecipitation reduction[10, 13-14]. The possible oxide existing on the surface of nano particles should be responsible for the unconventionality. Due to the large specific surface area of SnxSbNi, it is readily to be contaminated by oxygen during the preparation process. In the first lithiation phase, the oxides will be reduced to yield Li2O, resulting in the irreversible capacity, as Li cannot be extracted from Li2O during the electrochemistry reaction[13]. Moreover, the formation of SEI film on the electrode surface may also make contribution to the high initial capacity of Si film[15].
Cyclic voltammetry(CV) plots of Sn2SbNi alloy electrode for the first and the following 2 cycles are shown in Fig.6. In the initial discharge, the potential drops rapidly to 0.8 V, then decreases to 0.01 V gradually. The difference between the 1st and 2nd cycle is considered to result from the inactive features of alloy composite against the electrochemical reactions, and the decomposition of oxides on the particle surface during the first discharge process. From the second cycle, the CV profiles of batteries become almost overlapping in the following cycles evidently, illuminating the excellent durability of Sn2SbNi alloy composite electrode. The CV plots show four pairs of peaks, corresponding to the four potential plateaus in charge/discharge curves. For lithiation process, SEI film will form firstly on the particle surface before A during the first cycle, and always starts at about 1.0 V. Peak A at around 0.8 V corresponds to the formation of Li3Sb, while the peaks B, C and D represents the potential dependent formation of various Li-Sn alloys, because Sn can form different Li-Sn phases with Li. For delithiation process, these insertion processes are reversible. Li will first be extracted from the Li-Sn alloy (D′, C′ and B′), then from Li-Sb alloy (A′). The Sn2SbNi alloy composite electrode will be resumed when it is recharged to 1.2 V. However, the formation of the SEI film is irreversible.
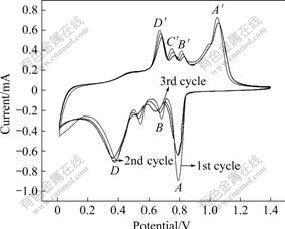
Fig.6 Plots of cyclic voltammetry of Sn2SbNi composite electrode for the first 3 cycles(Cut-off voltage of 0.1-1.5 V)
4 Conclusions
1) Nanosized SnxSbNi alloy composite anode powder
was fabricated by reduction precipitation reduction. The nano structure can accommodate the volume change caused by the lithiation/delithiation of electrode. The multi-element characteristics of active material allow the volume change to take place in a stepwise manner. The ductile component Ni will play as a buffer to relieve the volume change stress of electrode. All these factors contribute greatly to the excellent cycling stability of SnxSbNi alloy electrode.
2) Increasing the Sn content in SnxSbNi composite powder can increase the capacity of alloy electrode; however, excessive Sn may cause the fast electrode fading. Sn2SbNi electrode shows a high reversible specific capacity and an excellent cyclic ability.
References
[1] IDOTA Y, KUBOTT. MATSUFUJI A, MAEKAWA Y. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material[J]. Science, 1997, 276: 1395-1397.
[2] WANG Y, LEE J Y, DEIVARA T C. Tin nanopartical loaded graphite anodes for Li-ion battery[J]. J Electrochem Soc, 2004, 151(11): A1804-A1809.
[3] HU R Z, ZHANG L J, LIUX, ZENG M Q. Investigation of immiscible alloy system of Al-Sn thin films as anodes for lithium ion batteries[J]. Electrochem Commun, 2008, 10(7): 1109-1112.
[4] ZHAO H, YIN C, GUO H. Microcrystalline SnSb alloy powder as lithium storage material for rechargeable lithium-ion batteries[J]. Electrochem Solid-State Lett, 2008, 9(6): A281-A284.
[5] GUO H, ZHAO H, JIA X. Spherical Sn-Ni-C alloy anode material with submicro/microcomplex particle structure for lithium secondary batteries[J]. Electrochem Commun, 2007, 9(9): 2207-2212.
[6] DONG Q F, WU Z, JIN M G. Preparation and performance of nickel-tin alloys used as anodes for lithium-ion battery[J]. Solid State Ionics, 2004, 167: 49-54.
[7] WU Y P, RAHM E, HOLZE R. Carbon anode materials for lithium ion batteries[J]. J Power Sources, 2004, 114: 228-236.
[8] CHENG X Q, SHI P F. Electroless Cu-plated Ni3Sn4 alloy used as anode material for lithium ion battery[J]. J Alloys Compd, 2005, 391(1/2): 241-245.
[9] BEATTIE S D, DAHN J R. SnCux alloy anode prepared by chemical deposition reduction for lithium batteries[J]. J Electrochemical Society, 2003, 150(7): A894-A898.
[10] WACHER M, WINTER M, BESENHARD J O. Anodic materials for rechargeable Li-batteries[J]. J Powder Sources, 2002, 105: 151-160.
[11] LIOA Z X, MA F Z, HU J H. SnNi-deposited carbonaceous mesophase spherule as anode material for lithium ion batteries[J]. Electrochem Commun, 2005, 5(8): 657-661.
[12] KEPLE K D, AUGHEY J, THACKEYAY M M. Copper-tin anodes for rechargeable lithium batteries: An example of the matrix effect in an intermetallic system[J]. J Power Sources, 1999, 81: 383-387.
[13] CHEN L B, LI G Y. Influence of Sb on IMC growth in Sn-Ag-Cu-Sb Pb-free solder joints in reflow process[J]. Thin Solid Films, 2004, 462: 395-401.
[14] WACHTLER M, BESENHARD J O, WINTER M. Tin and tin-based intermetallics as new anode materials for lithium-ion cells[J]. J Power Sources, 2001, 94: 189-193.
[15] LINDSAY M J, WANG G X, LIU H K. Al-based anode materials for Li-ion batteries[J]. J Power Sources, 2003, 119: 84-87.
___________________________
Foundation item: Project(2008cd148) supported by the Social Development Plan of Yunnan Province, China; Project (2010) supported by Key Science and Technology Fund of Education Department, China
Corresponding author: GUO Hong; Tel: +86-871-5033679; Fax: +86-871-5033726; E-mail: guohongcom@126.com
(Edited by YANG Hua)