利用热分析技术评估碳质孕育Mg-3%Al合金熔体的凝固特性
来源期刊:中国有色金属学报(英文版)2018年第4期
论文作者:杜军 石裕同 李文芳
文章页码:812 - 818
关键词:镁铝合金;晶粒细化;碳质孕育;凝固特性;计算机辅助冷却曲线分析
Key words:Mg-Al alloy; grain refinement; carbon inoculation; solidification characteristics; computer-aided cooling curve analysis
摘 要:将Mg-3%Al合金熔体进行碳质孕育处理并保温不同时间,评估保温时间对孕育细化效果的影响。利用计算机辅助热分析技术对经碳质孕育并保温不同时间合金熔体的凝固特性进行分析。结果表明:碳质孕育能显著细化Mg-3%Al合金。当保温时间延长至60 min时孕育衰退不明显。碳质孕育能明显改变合金熔体的冷却曲线,孕育处理后初始形核温度和最低形核温度升高,再辉过冷度和再辉时间几乎降低至0。碳质孕育的晶粒细化效果能通过冷却曲线形状和凝固特征参数进行评估,包括初始形核温度、最低形核温度、再辉过冷度和再辉时间。
Abstract: The Mg-3%Al melt was inoculated by carbon with different holding time. The effect of holding time on grain refining efficiency was evaluated. The solidification characteristics of the carbon-inoculated Mg-3%Al melt with different holding time were assessed by computer-aided cooling curve analysis. The results showed that Mg-3%Al alloy could be effectively refined by carbon inoculation. Slight fading phenomenon occurred with increasing the holding time to 60 min. Carbon inoculation could significantly influence the shape of cooling curves of Mg-3%Al melt. The nucleation starting and minimum temperatures increased. The recalescence undercooling and duration decreased to almost zero after carbon inoculation. The grain refining efficiency of carbon inoculation could be assessed by the shape of the cooling curve and solidification characteristic parameters including nucleation starting and minimum temperatures, recalescence undercooling and duration.
Trans. Nonferrous Met. Soc. China 28(2018) 812-818
Jun DU, Yu-tong SHI, Wen-fang LI
School of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China
Received 13 December 2016; accepted 7 July 2017
Abstract: The Mg-3%Al melt was inoculated by carbon with different holding time. The effect of holding time on grain refining efficiency was evaluated. The solidification characteristics of the carbon-inoculated Mg-3%Al melt with different holding time were assessed by computer-aided cooling curve analysis. The results showed that Mg-3%Al alloy could be effectively refined by carbon inoculation. Slight fading phenomenon occurred with increasing the holding time to 60 min. Carbon inoculation could significantly influence the shape of cooling curves of Mg-3%Al melt. The nucleation starting and minimum temperatures increased. The recalescence undercooling and duration decreased to almost zero after carbon inoculation. The grain refining efficiency of carbon inoculation could be assessed by the shape of the cooling curve and solidification characteristic parameters including nucleation starting and minimum temperatures, recalescence undercooling and duration.
Key words: Mg-Al alloy; grain refinement; carbon inoculation; solidification characteristics; computer-aided cooling curve analysis
1 Introduction
As the lightest metallic structural materials, magnesium and its alloys are well established materials for cast parts because of their high specific strength and stiffness. They have wide application prospects in the automobile, railway and aviation industries, etc [1,2]. However, wider applications are limited due to poor ductility and formability of magnesium alloys. Improvement in ductility and formability is therefore desirable. It is well known that grain size is regarded as the key microstructural factor affecting nearly all aspects of the physical and mechanical properties of polycrystalline metallic products. As for magnesium alloys, grain refinement is a very effective method to improve the mechanical properties due to their high Hall-Petch coefficient [3-6]. In various magnesium alloys, Mg-Al-based alloys are the most common and economic commercial ones for which many grain refining methods have been developed, such as melt superheating [7,8], Elefinal process [9], melt agitation [10-12], alloying treatment [6,13,14] and carbon inoculation [15-17]. Among them, carbon inoculation has the advantages in high efficiency, low operating temperature and less fading [7-17].
Without doubt, optical microscopy is the best technique to investigate grain changes induced by grain refining treatment. The main drawbacks of this technique are the long time required to prepare a sample and the fact that the casting must be destroyed. Moreover, the quality of melt cannot be monitored on-line after being inoculated or modified. To date, computer-aided cooling curve analysis (CA-CCA) has been developed as a fast, non-destructive and in-situ method to evaluate the quality of the molten metal during melt treatment prior to casting. Compared with other thermal analysis (TA) methods, such as differential scanning calorimetry (DSC), differential thermal analysis (DTA) and thermogravimetric analysis (TGA), CA-CCA method is simple and most importantly suitable for commercial applications.
CA-CCA has been widely utilized in assessing the level of grain refinement and modification [18], level of impurity [19], interaction between addition elements [20,21] and optimizing refiner and modifier level in a foundry [22,23]. The precise shape of the cooling curve is directly related to the solidification and microstructural characteristics. KROHN [24] firstly proposed a new concept of metallurgical thumbprint, i.e., the precise shape of the cooling curve is significant with individual character as the human thumbprint for different molten melts. Previously, the CA-CCA method was mainly used in aluminum and gray iron foundries [18-28]. For example, SHABESTARI and MALEKAN [23] assessed the effect of Al-5Ti-B refiner on solidification characteristics of 319 aluminum alloy by using CA-CCA. Solidification parameters of nucleation and growth temperatures were improved after being refined. FARAHANY et al [25] evaluated the effect of Si, Sb and Sr on eutectic phases in ADC12 alloy by CA-CCA. The addition of Sb and Sr increased the nucleation temperature of Al2Cu, but the addition of Bi produced an opposite effect. CA-CCA was utilized by STAN et al [27] to monitor the quality of hypoeutectic cast irons during solidification in sand and metal moulds. Thermal analysis parameters, especially referring to the metastable eutectic temperature, could indicate the chill tendency of irons solidified in different conditions. In recent years, there have been reports on the use of CA-CCA method in order to characterize the solidification behavior of Mg alloys [29-32]. LIANG et al [29] disclosed the solidification pathways of Mg-Al-Ca system alloys by using CA-CCA.
In the above studies, most of them were carried out to disclose the effect of refiners on grain refinement of Al alloys by CA-CCA [18,19,21-23]. However, only a few studies were performed to assess the effect of grain refinement on solidification characteristics of Mg alloys [33,34]. In the study performed by GAO et al [33], the correlation of recalescence with grain refinement of Mg alloys was investigated and the results showed that the undercooling and duration of recalescence decreased after being refined by MgCO3. As for the carbon inoculation, less-fading was regarded as one of the important advantages. In our previous study, slight fading effect was found for the Mg-3%Al alloy inoculated by graphite [35]. However, the effect of holding time after being inoculated on solidification characteristics was not investigated. The present study mainly aimed to investigate the solidification characteristics of carbon- inoculated Mg-3%Al alloy with different holding time by using CA-CCA. The change of nucleating potency of nuclei with holding time was discussed based on the first derivative of cooling curve.
2 Experimental
2.1 Melt preparation
Mg-3%Al (mass fraction, the same below) alloy melt was prepared by melting high purity Mg (>99.95%) and Al (99.99%) ingots in MgO crucible of d60 mm × 100 mm in an electrical resistance furnace. Carbon inoculation was operated by introducing the carbon- containing pellets into the Mg-3%Al melt. The addition amount of carbon was 0.2% of the experimental material. The pellet was prepared by compacting the Mg, Al and graphite powders with a mass ratio of 5:4:1 by hydraulic pressure. The Mg melt was protected to avoid being oxidized with the gas mixture of 1% SF6 and 99% N2. The melting temperature and operating temperature of carbon inoculation were both 760 °C. The mass of Mg-3%Al alloy was about 250 g. About 50 g melt was poured into the cylindric iron-mould preheated at 500 °C to prepare the sample for grain observation. The rest melt in the crucible was used for thermal analysis.
Four samples were prepared in the present study. One sample was Mg-3%Al alloy without carbon inoculation. The rest three samples were the carbon-inoculated Mg-3%Al melt with different holding time of 20, 30 and 60 min to investigate the influence of holding time on grain refinement and solidification characteristics. The carbon-inoculated melt with different holding time was stirred to make them more uniform under the same condition. The holding time began to be recorded after the melt was stirred.
2.2 Thermal analysis
After being poured to prepare the sample for metallographic observation, the crucible containing the rest Mg-3%Al melt was placed in another home-made electrical resistance furnace with cylindrical cavity, as shown in Fig. 1. This electrical resistance furnace was utilized to slower the cooling rate and keep the melt cooling at the same cooling rate of approximately 0.3 °C/s. Before the crucible was placed into this furnace, the temperature at the center of the furnace was set constantly to be 350 °C. The furnace was switched off when the crucible was placed in it. Three shielded K-type thermocouples were inserted in the center of the crucible at the same depth in the melt (30 mm from the bottom) to measure temperature-time data of the melt as it cooled. The data were recorded by using national instruments with high-speed data acquisition system connected to a computer with Labview software.
2.3 Microstructure observation
The cylindrical ingot with the diameter of d20 mm was prepared. Metallographic samples were cut in the horizontal direction at the position of 10 mm from the bottom of the samples. The samples were ground by SiC abrasive paper and mechanically polished. After that, the samples were chemically polished with 10% HNO3 ethanol solution firstly and then etched with picric acid solution to reveal grain boundary. The Leica DFC320 type optical microscope and linear intercept method described in ASTM standard E112-88 were used to observe and evaluate the grain size.
3 Results
3.1 Grain refining efficiency
Figure 2 shows the grain morphologies of Mg-3%Al alloy without treatment and treated by carbon inoculation with different holding time. As for the sample without treatment, its grain was coarse with average grain size of (510±50) μm (Fig. 2(a)). The grain could be obviously refined by carbon inoculation, as shown in Figs. 2(b)-(d). The grain sizes were remarkably refined to (189±14) and (199±16) μm with the holding time of 20 and 30 min, respectively. With prolonging the holding time to 60 min, the grain slightly became coarse with the size of (230±20) μm. Figure 3 shows the effect of holding time on grain size of Mg-3%Al alloy refined by carbon inoculation. Slight fading phenomenon occurred with increasing the holding time to 60 min.
3.2 Cooling curve thermal analysis
Figure 4 shows the typical cooling curves of the Mg-3%Al alloy without treatment and treated by carbon inoculation with the holding time of 20 min. The data acquisition was stopped at about 600 °C since no obvious phase transformation occurs for Mg-3%Al alloy below this temperature. The average cooling rate was 0.25-0.29 °C/s before solidification.
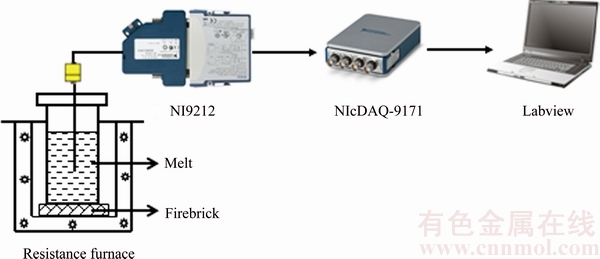
Fig. 1 Experimental configuration of thermal analysis
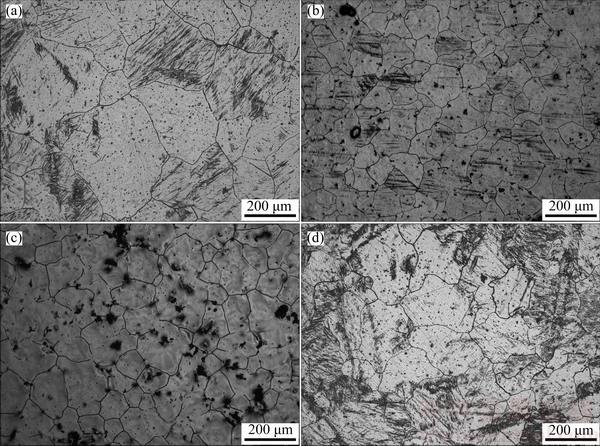
Fig. 2 Grain morphologies of Mg-3%Al alloy without treatment (a) and treated by carbon inoculation with different holding time of 20 min (b), 30 min (c) and 60 min (d)
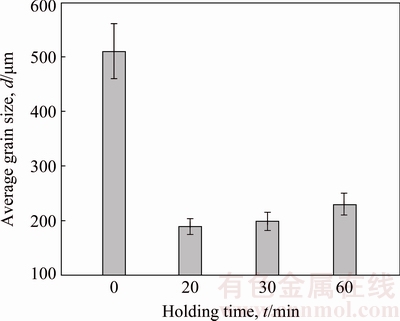
Fig. 3 Effect of holding time on grain size of carbon-inoculated Mg-3%Al alloy
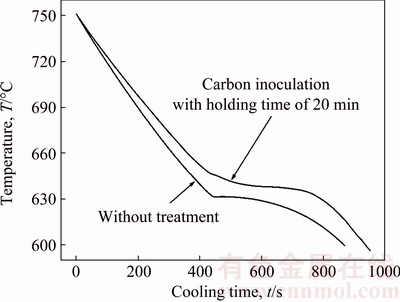
Fig. 4 Typical cooling curves of Mg-3%Al alloy without treatment and treated by carbon inoculation with holding time of 20 min
Figure 5 illustrates the cooling curve of the Mg-3%Al alloy without treatment and its first derivative curve (dT/dt) during the solidifying range of 625-635 °C to obtain the detailed solidification characteristics. The first derivative of the cooling curve was used to enhance gradient changes related to the phase transformation and to precisely determine the critical solidification characteristics of the alloys. Some critical points of the cooling curve were identified as characteristic temperatures, namely Ts, Tmin and Tr [31,32]. Ts is the initial nucleation starting temperature of primary α-Mg phase. Tmin is the nucleation minimum temperature, beyond which the latent heat generation of the newly nucleated crystals exceeds the heat emission to vicinity. Tr is the stable recalescence temperature due to the release of latent heat of primary α-Mg nucleation. Ts is determined from the inflection point of the first derivative curve. Tmin and Tr are determined from the zero points of the first derivative curve [31,32]. ΔTrec, that is, Tr-Tmin, is the recalescence undercooling. trec is the duration of recalescence. As for the Mg-3%Al alloy, its temperatures Ts and Tmin were (634±0.52) °C and (631.4±0.17) °C, respectively. The recalescence under- cooling ΔTrec was only 0.2 °C and the duration of recalescence trec is about 20 s.
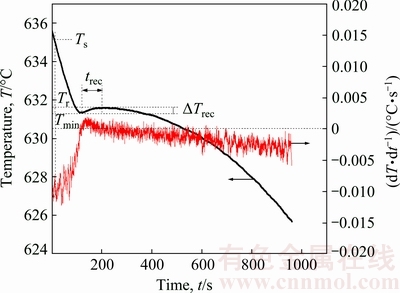
Fig. 5 Cooling curve and its first derivative of Mg-3%Al alloy without treatment labeled with characteristic point
To compare the detailed solidification processes of the sample without/with carbon inoculation, Fig. 6 shows the cooling curves of the all samples in the temperature range of 620-650 °C. It can be obviously seen that carbon inoculation shifts the cooling curves upward with increasing the nucleation temperature. Besides, no obvious recalescence occurred for the Mg-3%Al alloy after being carbon-inoculated.
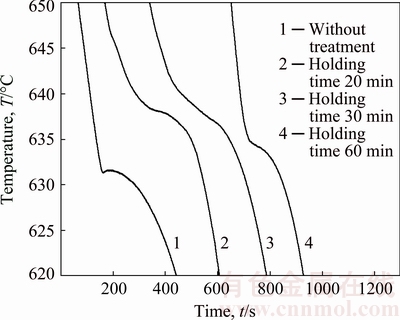
Fig. 6 Cooling curves of Mg-3%Al alloy without treatment and treated by carbon inoculation with different holding time of 20, 30 and 60 min
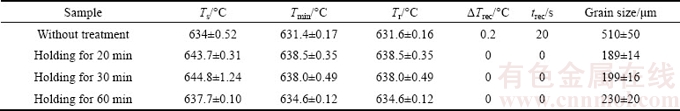
The temperatures of Ts and Tmin could be obtained from the first derivative curves of the cooling curves for the carbon-inoculated samples with different holding time, as listed in Table 1. For the Mg-3%Al alloy inoculated by carbon with the holding time of 20 min, the nucleation starting temperature Ts and the nucleation minimum temperature Tmin were raised to (643.7±0.31) °C and (638.5±0.35) °C, respectively. Ts and Tmin were almost the same although the holding time was prolonged from 20 to 30 min. They were (644.8±1.24) and (638.0±0.49) °C, respectively. However, Ts and Tmin were declined to (637.7±0.10) and (634.6±0.12) °C when the holding time was prolonged to 60 min. It should be noted that the recalescence undercooling ΔTrec was almost 0 °C and the duration of recalescence trec was 0 s since no obvious recalescence occurred.
Table 1 Parameters of solidification characteristics of Mg-3%Al alloy without treatment and treated by carbon inoculation with different holding time
4 Discussion
It is well known that the shape of the cooling curve is the integrated result of the heat elimination to the surroundings and the latent heat generation in the mold during solidification. The shape of the cooling curve is the reflection of solidification characteristics. Known from the above results, carbon inoculation significantly influenced the solidification characteristics of Mg- 3%Al alloy. The initial nucleation starting temperature was remarkably improved from (634±0.52) to (643.7±0.31) °C after being carbon-inoculated with the holding time of 20 min. Also, the nucleation minimum temperature was improved from (631.4±0.17) to (638.5±0.35) °C. With prolonging the holding time to 60 min, the nucleation starting and minimum temperatures were reduced. However, these temperatures were still higher than those of Mg-3%Al alloy without treatment. Moreover, it should be noted that the recalescence undercooling temperature decreased to zero after carbon inoculation. Prolonging the holding time did not affect the recalescence undercooling temperature significantly.
It could be seen that there exists an obvious relationship between average grain size and parameters of solidification characteristics, as listed in Table 1. Carbon inoculation resulted in grain refinement and the increase of Ts, Tmin and Tr. With prolonging the holding time to 60 min, the grain size increased whilst the Ts, Tmin and Tr decreased. Consequently, the solidification characteristics and the shape of cooling curve could be used to quantitatively assess the grain refinement efficiency of Mg-Al alloy by carbon inoculation.
As for refining mechanism by carbon inoculation, it has been widely accepted that Al4C3 particles formed by the reaction of Al and C in the alloy melt act as potent nuclei for primary α-Mg grains based on experimental results and in view of crystallography [7-13]. Al4C3 (a=0.3335 nm, c=2.4967 nm) has a crystallographic similarity to a-Mg (a=0.3209 nm, c=0.5211 nm) [36,37]. In some researches [14,15], Al4C3 was directly proved as an effective refiner to Mg-Al alloys since Al4C3 powders or Al4C3-containing master alloys were used.
Under the condition of homogeneous nucleation during melt solidification, the formation of stable nuclei needs large undercooling. The initial nucleation starting temperature is low, as the sample without treatment listed in Table 1. After a large number of homogeneous nuclei form and grow, the latent heat generation will result in the increase of temperature, i.e., recalescence. The increase of temperature will inhibit the formation of new nuclei. Relatively, the growth of a small amount of nuclei results in coarsening of grains. After being carbon-inoculated for Mg-3%Al melt, the formed Al4C3 particles could stably exist in the Mg-Al melt since its melt point is higher than 2000 °C [38]. The nucleation cooling of α-Mg was decreased due to the existence of many potent nucleating Al4C3 particles. The initial nucleation starting temperature increased, as listed in Table 1. The formation and grain growth by a large amount of nuclei will result in much solidification latent heat. The nucleation minimum temperature Tmin should also be improved. Consequently, the cooling rate and the recalescence cooling during solidification stage will decrease, i.e., ΔTrec→0 °C and trec→0 s.
Known from Fig. 3, slight fading phenomenon occurred with increasing the holding time to 60 min. The effect of holding time on grain refinement efficiency was discussed in our previous study [35]. The optimal grain refining efficiency could be obtained when the holding time was 20-30 min. With increasing in the holding time, the potency of nucleating Al4C3 particles could be decreased resulting from the following factors. One is the settling of Al4C3 particles due to the density difference between Al4C3 (2.36 g/cm3) and Mg melt (1.7 g/cm3). The other is that the surface activity of the Al4C3 particles could be disturbed by other elements, such as Fe and Mn [7,39]. Consequently, nucleation cooling should be improved and then the nucleation starting temperature and minimum temperature decreased.
CA-CCA has been commercially used to monitor the grain refinement and modification of aluminum castings since solidification characteristics can reflect the nucleation potential of the melt. This study mainly aimed to investigate the solidification characteristics of carbon- inoculated Mg-3%Al alloy with different holding time. Critical temperature corresponded to the grain refinement efficiency with different holding time. The solidification characteristics of the alloy melt were influenced by heterogeneous nucleation. It was suggested that with the increase of ΔTrec the nucleation potency of nuclei decreased. When an alloy melt contains few favorable heterogeneous nucleation sites, high undercooling temperature will be needed to activate impotent nuclei for nucleation process and finally a coarse-grain structure was accessed. When an alloy melt has sufficient favorable heterogeneous nucleation sites, small undercooling temperature or no undercooling will be needed and solidification will start at higher temperature. Finally, the alloy has a fine grain size.
In this study, carbon inoculation was confirmed to effectively refine the grain size of Mg-3%Al alloy regardless of holding time and to affect solidification characteristics. The carbon agent gave rise to sufficient favorable heterogeneous nucleation sites, which led to the significant grain refinement and the change of solidification characteristics.
5 Conclusions
1) Mg-3%Al alloy could be effectively refined by carbon inoculation. Slight fading phenomenon occurred with increasing the holding time to 60 min.
2) Carbon inoculation could significantly influence the shape of cooling curves and the recalescence of the Mg-3%Al alloy. The nucleation starting and minimum temperatures increased. The recalescence undercooling and duration were decreased to almost zero after carbon inoculation.
3) The grain refining efficiency of carbon inoculation could be assessed by the shape of the cooling curve and solidification characteristic temperatures, including nucleation starting and minimum temperatures, recalescence undercooling and duration.
References
[1] LUO A A. Fundamentals of magnesium alloy metallurgy [M]. Cambridge: Woodhead Publishing, 2013: 266-316.
[2] LUO A A. Magnesium casting technology for structural applications [J]. Journal of Magnesium and Alloys, 2013, 1(1): 2-22.
[3] PAN Hu-cheng, REN Yu-ping, FU He, ZHAO Hong, WANG Li-qin, MENG Xiang-ying, QIN Gao-wu. Recent developments in rare-earth free wrought magnesium alloys having high strength: A review [J]. Journal of Alloys and Compounds, 2016, 663: 321-331.
[4] KASEEM M, CHUNG B K, YANG H W, HAMAD K, KO Y G. Effect of deformation temperature on microstructure and mechanical properties of AZ31 Mg alloy processed by differential-speed rolling [J]. Journal of Material Science Technology, 2015, 31(5): 498-503.
[5] FARAJI G, YAVARI P, AGHDAMIFAR S, MASHHADI M M. Mechanical and microstructural properties of ultra-fine grained AZ91 magnesium alloy tubes processed via multi pass tubular channel angular pressing (TCAP) [J]. Journal of Material Science and Technology, 2014, 30: 134-138.
[6] ZENG Xiao-qin, WANG Ying-xin, DING Wen-jiang, LUO A A, SACHDEV A K. Effect of strontium on the microstructure, mechanical properties, and fracture behavior of AZ31 magnesium alloy [J]. Metallurgical Materials Transactions A, 2006, 37(4): 1333-1341.
[7] CAO P, QIAN M, STJOHN D H. Mechanism for grain refinement of magnesium alloys by superheating [J]. Scripta Materialia, 2007, 56(7): 633-636.
[8] MOTEGI T. Grain–refining mechanisms of superheat-treatment of and carbon addition to Mg-Al-Zn alloys [J]. Materials Science and Engineering A, 2005, 413-414(S): s408-s411.
[9] CAO P, QIAN M, STJOHN D H. Effect of iron on grain refinement of high-purity Mg-Al alloys [J]. Scripta Materialia, 2004, 51(2): 125-129.
[10] LIU Xin-bao, OSAWA Y, TAKAMORI S, MUKAI T. Grain refinement of AZ91 alloy by introducing ultrasonic vibration during solidification [J]. Materials Letters, 2008, 62: 2872-2875.
[11] FAN Z, WANG Y, XIA M, ARUMUGANATHAR S. Enhanced heterogeneous nucleation in AZ91D alloy by intensive melt shearing [J]. Acta Materialia, 2009, 57(16): 4891-4901.
[12] BHINGOLE P P, CHAUDHARI G P. Synergy of nanocarbon black inoculation and high intensity ultrasonic processing in cast magnesium alloys [J]. Materials Science and Engineering A, 2012, 556: 954-961.
[13] ALI Y, QIU Dong, JIANG Bin, PAN Fu-sheng, ZHANG Ming-xing. Current research progress in grain refinement of cast magnesium alloys: A review article [J]. Journal of Alloys and Compounds, 2015, 619: 639-651.
[14] LEE Y C, DAHLE A K, STJOHN D H. The role of solute in grain refinement of magnesium [J]. Metallurgical Materials Transactions A, 2000, 31: 2895-2906.
[15] DU Jun, YANG Jian, KUWABARA M, LI Wen-fang, PENG Ji-hua.Effects of carbon and/or alkaline earth elements on grain refinement and tensile strength for AZ31 alloy [J]. Materials Transactions, 2008, 49(10): 2303-2309.
[16] SURESH M, SRINIVASAN A, RAVI K R, PILLAI U T S, PAI B C. Microstructural refinement and tensile properties enhancement of Mg-3Al alloy using charcoal additions [J]. Materials Science and Engineering A, 2011, 528(6): 2502-2508.
[17] WANG Ling, KIM Y M, LEE J H, YOU B S. Effect of magnesium carbonate on microstructure and rolling behaviors of AZ31 alloy [J]. Materials Science and Engineering A, 2011, 528(3): 1485-1490.
[18] APELIAN D, SIGWORTH G K, WHALER K R. Assessment of grain refinement and modification of Al-Si foundry alloys by thermal analysis [J]. AFS Transactions, 1984, 92: 297-307.
[19] MAHFOUD M, RAO A K P, EMADI D. The role of thermal analysis in detecting impurity levels during aluminum recycling [J]. Journal of Thermal Analysis and Calorimetry, 2010, 100(3): 847-851.
[20] LU L, DAHLE A K. Effects of combined additions of Sr and AlTiB grain refiners in hypoeutectic Al-Si foundry alloys [J]. Materials Science and Engineering A, 2006, 435-436(S): s288-s296.
[21] LUDWIG T H, DALEN E S, SCHAFFER P L, ARNBERG L. The effect of Ca and P interaction on the Al-Si eutectic in a hypoeutectic Al-Si alloy [J]. Journal of Alloys and Compounds, 2014, 586(6): 180-190.
[22] FARAHANY S, OURDJINI A, IDRIS M H. The usage of computer-aided cooling curve thermal analysis to optimize eutectic refiner and modifier in Al-Si alloys [J]. Journal of Thermal Analysis and Calorimetry, 2012, 109(1): 105-111.
[23] SHABESTARI S G, MALEKAN M. Assessment of the effect of grain refinement on the solidification characteristics of 319 aluminum alloy using thermal analysis [J]. Journal of Alloys and Compounds, 2010, 492: 134-142.
[24] KROHN B R. Thermal analysis: Metallurgical thumbprint [J]. Modern Casting, 1985, 75: 21-25.
[25] FARAHANY S, OURDJINI A, IDRIS M H, SHABESTARI S G. Evaluation of the effect of Bi, Sb, Sr and cooling condition on eutectic phases in an Al-Si-Cu alloy (ADC12) by in situ thermal analysis [J]. Thermochimica Acta, 2013, 559(5): 59-68.
[26] FARAHANY S, OURDJINI A, IDRIS M H, SHABESTARI S G. Computer-aided cooling curve thermal analysis of near eutectic Al-Si-Cu-Fe alloy [J]. Journal of Thermal Analysis and Calorimetry, 2013, 114(2): 705-717.
[27] STAN S, CHISAMERA M, RIPOSAN I, BARSTOW M. Application of thermal analysis to monitor the quality of hypoeutectic cast irons during solidification in sand and metal moulds [J]. Journal of Thermal Analysis and Calorimetry, 2012, 110(3): 1185-1192.
[28] STEFANESCU D M. Thermal analysis-theory and applications in metal casting [J]. International Journal of Metal Casting, 2015, 9(1): 7-22.
[29] LIANG Song-mao, CHEN Rong-shi, BLANDIN J J, SUERY M, HAN E H. Thermal analysis and solidification pathways of Mg-Al-Ca system alloys [J]. Materials Science and Engineering A, 2008, 480: 365-372.
[30] HUANG Zhang-hong, LIANG Song-mao, CHEN Rong-shi, HAN E H. Solidification pathways and constituent phase of Mg-Zn-Y-Zr alloys [J]. Journal of Alloys and Compounds, 2009, 468: 170-178.
[31] FARAHANY S, BAKHSHESHI-RAD H R, IDRIS M H, KADIR M R A, LOTFABADI A F, OURDJINI A. In-situ thermal analysis and macroscopical characterization of Mg-xCa and Mg-0.5Ca-xZn alloy systems [J]. Thermochimica Acta, 2012, 527(1): 180-189.
[32] PANG Song, WU Guo-hua, LIU Wen-cai, ZHANG Liang, ZHANG Yang, CONRAD H, DING Wen-jiang. Influence of cooling rate on solidification behavior of sand-cast Mg-10Gd-3Y-0.4Zr alloy [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3413-3420.
[33] GAO Lei, LIANG Song-mao, CHEN Rong-shi, HAN E H. Correlation of recalescence with grain refinement of magnesium alloys [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(S): s288-s291.
[34] MEN H, JIANG B, FAN Z. Mechanisms of grain refinement by intensive shearing of AZ91 alloy melt [J]. Acta Materialia, 2010, 58(19): 6526-6534.
[35] DU Jun, WANG Ming-hua, ZHOU Ming-chuan, LI Wen-fang. Evolutions of grain size and nucleating particles in carbon-inoculated Mg-3%Al alloy [J]. Journal of Alloys and Compounds, 2014, 592(14): 313-318.
[36] CAO P, QIAN M, STJOHN D H. Native grain refinement of magnesium alloys [J]. Scripta Materialia, 2005, 36(7): 841-844.
[37] HAN Guang, LIU Xiang-fa, DING Hai-min. Grain refinement of Mg-Al based alloys by a new Al-C master alloy [J]. Journal of Alloys and Compounds, 2008, 467(1): 202-207.
[38] MASSALSKI T B, OKAMOTO H, SUBRAMANIAN P R, KACPRZAK L. Binary alloy phase diagrams [M]. Clevand: ASM International, 1986.
[39] HAITANI T, TAMURA Y, YANO E, MOTEGI T, KONO N, SATO E. Grain refining mechanism of high-purity Mg-9mass%Al alloy ingot and influence of Fe or Mn addition on cast grain size [J]. Journal of Japan Institute of Light Metals, 2001, 51: 403-408.
杜 军,石裕同,李文芳
华南理工大学 材料科学与工程学院,广州 510640
摘 要:将Mg-3%Al合金熔体进行碳质孕育处理并保温不同时间,评估保温时间对孕育细化效果的影响。利用计算机辅助热分析技术对经碳质孕育并保温不同时间合金熔体的凝固特性进行分析。结果表明:碳质孕育能显著细化Mg-3%Al合金。当保温时间延长至60 min时孕育衰退不明显。碳质孕育能明显改变合金熔体的冷却曲线,孕育处理后初始形核温度和最低形核温度升高,再辉过冷度和再辉时间几乎降低至0。碳质孕育的晶粒细化效果能通过冷却曲线形状和凝固特征参数进行评估,包括初始形核温度、最低形核温度、再辉过冷度和再辉时间。
关键词:镁铝合金;晶粒细化;碳质孕育;凝固特性;计算机辅助冷却曲线分析
(Edited by Wei-ping CHEN)
Foundation item: Project (51574127) supported by the National Natural Science Foundation of China; Project (2014A030313221) supported by the Natural Science Foundation of Guangdong Province, China
Corresponding author: Jun DU; Tel/Fax: +86-20-87113597; E-mail: tandujun@sina.com
DOI: 10.1016/S1003-6326(18)64714-4

