DOI: 10.11817/j.ysxb.1004.0609.2020-35892
三氧化二铁在铜冶炼污酸中浸出行为及动力学
高一强,赵一萍,张利祥,黄建洪,田森林
(昆明理工大学 环境科学与工程学院,昆明 650500)
摘 要:通过研究不同温度下三氧化二铁(Fe2O3)在铜冶炼污酸中的浸出情况,考察了不同酸度污酸在720 min内的浸出性能和反应过程溶液pH值的变化,探讨了反应过程中的动力学和对污酸中砷的处理效果。结果表明:在整个反应的720 min内,溶液pH并无明显变化,Fe2O3在污酸中的浸出反应过程可以用收缩未反应核模型描述,在10~60 min的反应过程受固相层扩散控制比较明显,浸出反应表观活化能为8.1633 kJ/mol,而60~720 min的反应过程受化学反应过程控制比较明显,浸出反应表观活化能为43.0429 kJ/mol。同时,在65 ℃下Fe2O3对污酸中的砷处理效率可达90%,具有较好的除砷效果。
关键词:三氧化二铁;铜冶炼污酸;砷;浸出动力学
文章编号:1004-0609(2020)-10-2418-09 中图分类号:X751 文献标志码:A
铜冶炼污酸主要来源于烟气制酸过程的烟气洗涤过程与烟气制酸各工段[1-3]。目前大型烟气制酸装置的净化工段大部分都是采用湿法净化技术,在湿法净化过程均会产生污酸,该废水含酸量较高,并且成分复杂,是铜冶炼行业中较难处理的污染物之一。由于铜冶炼污酸废水酸度普遍较高(pH<1),且含有重金属等,直接排放于水体不仅会使水环境中的pH值发生改变,从而影响水生动植物的生态环境,破坏水体的自净能力[4-5],而其具有的高毒性和易于在生物体内富集特点,会严重威胁到人类健康的安全[6]。砷作为铜冶炼污酸中含量较高的的污染物,一般含量可达6 g/L。砷有剧毒在生物体内潜伏期长且具有致癌性,被美国疾病控制中心和国际防癌研究机构确定为第一类致癌物[7-9],一旦含砷过量的废水排放进环境中造成的危害是不可逆的。目前许多方法运用于处理污酸中的砷和重金属,包括化学沉淀法、吸附法、膜法和电化学方法[10-13]等,与其他的方法相比,化学沉淀法在企业中应用最为广泛。某铜业公司采用石灰中和沉淀法,在中和了污酸酸性的同时能够去除其中大部分砷和重金属污染物,但会产生大量的废渣,而这些废渣属于危险废物,不利于运输和处置。石灰—铁盐沉淀法是改进的化学沉淀法,具有操作方法简单、企业投资成本小、整体工艺运行费用小的优点。然而,这同样会产生大量的工业废渣,使其中的有价元素没有得到充分地利用,同时在酸性的污水环境中,一部分两性金属氢氧化物将会复溶[14]。
目前,对铁基化合物的研究,特别是关于铁基氧化物和氢氧化物在水污染治理中的应用研究成为近几年的研究热点。王强等[15]研究了赤铁矿对污水中砷的吸附解吸及氧化特征,表明赤铁矿对砷具有很强的吸附能力,在低pH值下赤铁矿对砷离子的去除主要是As(Ⅲ)的氧化和As(Ⅴ)为主的吸附共同作用的结果。DE MELLO等[16]研究了Al-Fe共沉淀物从废水中去除砷的效率并对产物的化学稳定性进行评估。关于矿物的反应浸出动力学,国内外研究者也做了大量工作。SENANYAKE等[17]研究通过在硫酸中通入SO2来实现褐铁矿石的还原溶解。JEONG等[18]利用冷冻浓缩效应在酸溶液中加入无机酸阴离子研究氧化铁的溶解情况。OLVERA-VENEGAS等[19]利用一种收缩颗粒核心模型来描述硫代硫酸钠和柠檬酸在高岭土中的浸出铁反应动力学,发现铁浸出受化学反应动力学控制。田庆华等[20]研究了盐酸体系中辉锑矿的臭氧协同氧化浸出动力学,徐志锋等[21]开展了富氧硫酸体系中硫化锌精矿的常压直接浸出动力学研究。前人[22-25]还对赤泥中铁的反应动力学进行研究,以未反应收缩核模型对其浸出过程进行拟合取得了较好效果。铁基氧化矿物众多,利用其处理污酸主要是将铁成分提取出来,通过吸附和化学沉淀的方法达到去除砷及重金属的效果。研究矿物的浸出动力学行为可以更好的利用其中的有用元素,使效益最大化。然而,目前关于铁基氧化物在污酸处理中的应用及浸出动力学行为研究报道还较少。
本工作探讨铁基氧化物三氧化二铁(Fe2O3)在污酸中的浸出动力学和对砷的去处效果,对不同温度下Fe2O3在污酸中的反应情况进行研究,并得出其反应动力学模型。此外,通过调节反应后溶液pH值,可利用Fe2O3去除铜冶炼污酸中的砷。本研究结果可以为改进污酸处理工艺、提高铁浸出率、实现铁基氧化物在污酸处理中的应用提供一定的参考。
1 实验
1.1 材料性质
试验所用Fe2O3 (69.80%~70.10%,质量分数,以Fe计)试剂来自天津致远化学试剂有限公司。Fe2O3粉末的XRD谱、FESEM像和EDS谱如图1所示。由图1(a)可以看出,原料中除含有Fe2O3还存在杂质SiO2,由图1(b)可以看出,原料质地较为密实,空隙较小。如图1(c)和表1所示,经过能谱分析,该Fe2O3原料中铁元素质量分数为69.81%。利用动态光散射对2 min内的Fe2O3粒径分布进行测定,波长532 nm,散射角为90°,实验温度为室温,得到Fe2O3平均粒径约为4.74 μm(见图1(d))。
1.2 试剂、仪器
试剂:浓硫酸、氢氧化钠等均为国产,分析纯,来自国药集团化学试剂有限公司。砷的国家标准溶液来自国家钢铁材料测试中心。
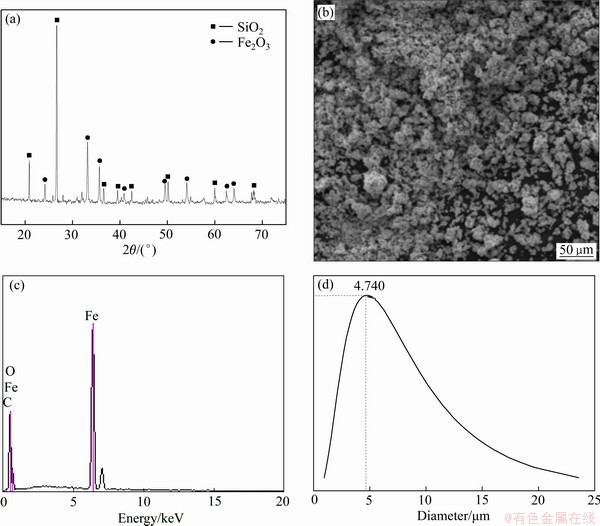
图1 Fe2O3原料的XRD谱、FESEM像、EDS谱和粒径分布图
Fig. 1 XRD pattern(a), field emission scanning electron microscope (FESEM)(b), energy dispersive spectrometer (EDS)(c) and particle size distribution(d) of Fe2O3 raw material
主要仪器:电子分析天平(美国奥斯豪公司生产);PHS-3C台式酸度计(上海虹益仪器仪表有限公司生产);恒温震荡箱(上海一恒科学仪器有限公司生产);TAS-992火焰原子分光光度计(北京普析通用仪器有限责任公司生产);AFS-8230原子荧光光度计(北京吉天仪器有限公司生产);101-I型电热鼓风干燥箱(成都志能实验仪器有限公司生产);场发射扫描电子显微镜(Hitachi S-4800型日立公司生产);X射线衍射仪(荷兰Per Pro MPD公司生产);动态光散射仪(BI-200SM,美国Brookhaven公司生产)。
表1 Fe2O3原料元素分析
Table 1 Elemental analysis of ferric oxide raw (mass fraction, %)
1.3 实验方法
本研究根据云南某铜业公司提供的铜冶炼污酸,经分析成分如表2所示,该酸度在80~200 g/L之间,因铜冶炼污酸成分复杂,试验采用相应酸度硫酸进行Fe2O3的浸出动力学研究,排除其他因素影响,同时研究在此酸度范围内反应溶液pH值的变化情况及对砷去除的效果。
表2 铜冶炼污酸成分分析
Table 2 Analysis of copper-smelting waste acid components

1) 温度及酸度对Fe2O3浸出铁的影响
分别设定酸度为80、120、150、180 g/L的污酸梯度,将4.36 g Fe2O3与100 mL污酸混合。在25、45和65 ℃下分别进行720 min反应实验,恒温震荡箱转速为200 r/min,反应结束后测定溶液pH及铁浓度。反应结束后将经过硫酸浸出后的Fe2O3在101-I型电热鼓风干燥箱内干燥(温度55 ℃,时间24 h)后制样,利用场发射扫描电子显微镜(FESEM)进行固体表面形态分析。
2) Fe2O3对污酸中砷的处理
用砷标准溶液配置含砷10 mg/L的污酸,与0.1 g Fe2O3在65 ℃下反应60 min,恒温震荡箱转速为200 r/min。反应结束后检测溶液pH,并用2.5 mol/L氢氧化钠调节溶液pH至4~5后,经0.45 μm滤膜过滤,得上清液测定砷含量和剩余铁含量。
在浸出反应过程中转化率(浸出率)X为总铁的浸出量与加入Fe2O3质量的比值,可表示为:
X=ρ·V/4.36 (1)
式中:X为反应物的转化率(浸出率),%;ρ为浸出液中总铁浓度,mg/L;V为浸出液体积,L。
2 结果与讨论
2.1 温度与酸度对三氧化二铁浸出的影响
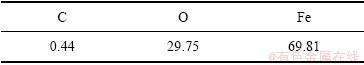
前期实验研究在25和65 ℃下不同浓度污酸和Fe2O3反应,在低浓度0.98 g/L至24.5 g/L污酸中,Fe2O3的浸出效果并不理想,在24.5 g/L反应600 min后Fe2O3的浸出率仅有1.03%,但可以看出随着酸度的增加,浸出的铁浓度增加。如图2所示,实验用实际污酸与相当酸度的硫酸进行模拟试验,结果显示Fe2O3浸出过程和浸出铁量并无显著差异。因此,在25 ℃下继续增加污酸浓度至180 g/L,其浸出铁浓度存在明显差异。在65 ℃下随着反应时间的延长,溶液中铁浓度逐渐增加,也可以看出,酸度越高,Fe2O3的浸出效率越高。但随着溶液中铁浓度的增加,氢离子的扩散系数降低,流动性下降[26],导致180 g/L和150 g/L污酸溶液中反应最后浸出铁浓度相差较小。由图3可以看出,在25 ℃和65 ℃下,污酸溶液中Fe2O3的浸出率存在显著差异。在室温25 ℃下,不同酸度的污酸对Fe2O3的浸出影响不同。在浓度为80~150 g/L范围内,随着酸度的升高,溶液中铁浓度增加,更多的氢离子参与到Fe2O3的溶解反应中,但当硫酸浓度增加至180 g/L时,溶液中铁浓度降至150 g/L时的1/2左右。当溶液中含有大量氢离子时,由于温度低,在反应开始时,Fe2O3表面出现类似钝化的现象[27],阻碍硫酸继续侵蚀其内部,导致Fe2O3不能持续浸出。

图2 配比污酸与硫酸浸出铁对比
Fig. 2 Comparison of leaching iron with copper-smelting waste acid and sulfuric acid
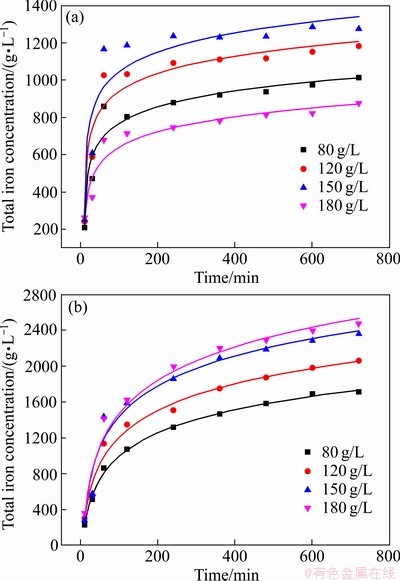
图3 25 ℃和65 ℃下不同酸度污酸溶液中浸出铁的变化
Fig. 3 Changes of iron dissolution in waste acid solution with different acidities at 25 ℃(a) and 65 ℃(b)
同时,在反应过程中,污酸溶液pH的变化情况如图4所示。由图4可见,在整个反应的720 min中,发现污酸溶液pH并不会发生激烈的变化,而一直维持在一个相对稳定的数值范围。这种现象在很多文献中都有提到,有些甚至出现下降的现象,但现在还没有一个经过验证的科学解释[28-29]。经过不同酸度污酸的侵蚀,Fe2O3形态发生一定的变化。如图5所示,Fe2O3由原来的紧密状态变为较疏松且形成一定的不规则侵蚀小孔,大多数铁元素仍然存在于Fe2O3晶格中,而未被释放出来,推测出Fe2O3还可以继续浸出,可进行反复浸出实验。
2.2 浸出动力学分析
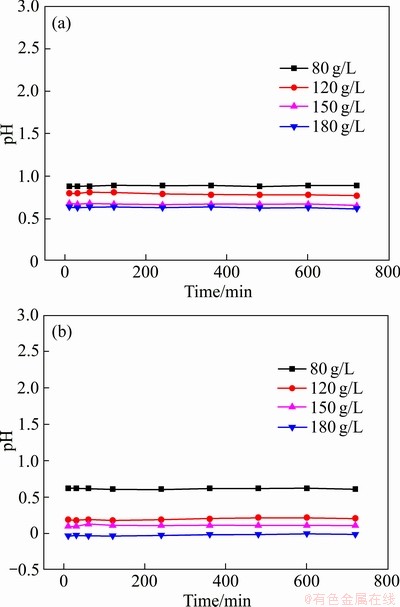
图4 25 ℃和65 ℃下污酸溶液pH的变化
Fig.4 Change of pH of waste acid solution at 25 ℃(a) and 65 ℃(b)
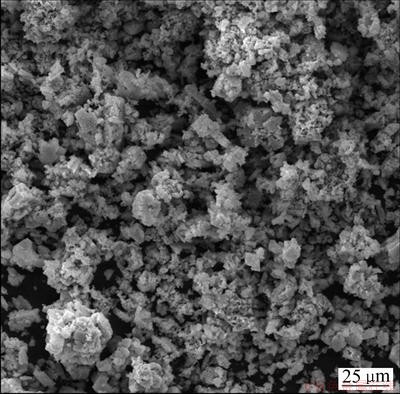
图5 反应720 min后Fe2O3的FESEM像
Fig. 5 Field emission scanning electron microscope (FESEM) image of Fe2O3 after reaction for 720 min
浸出动力学研究目的在于确定浸出速度与基本参数之间的关系[30-31],在本次研究中Fe2O3在污酸中的浸出过程中发生如下化学反应:
Fe2O3+3H2SO4=Fe2(SO4)3+3H2O
由上述反应方程可知,此反应过程属于液-固两相反应过程,宏观反应过程可用以下步骤进行描述:
1) 液相主体中的硫酸根离子和氢离子通过Fe2O3表面的液膜层向固体的表面扩散。
2) 扩散至固体表面的硫酸根离子和氢离子向Fe2O3内部扩散。
3) 硫酸根离子和氢离子与固相物料Fe2O3发生化学反应。
4) 反应生成的硫酸铁水合物通过Fe2O3固相向外部扩散。
5) 反应生成的硫酸铁水合物通过液膜层向液相主体进行扩散。
根据上述内容,在整个浸出过程中,反应物主体浓度基本恒定,可选择收缩未反应核模型进行描述。Fe2O3的浸出反应进程由液固界面上的化学反应步骤、液相反应物穿过固体外表面的液膜层和硫酸铁水合产物穿过未反应层的扩散步骤构成,整个反应过程可由化学反应步骤或者扩散步骤控制,或者两者共同控制。根据反应条件的不同,Fe2O3在污酸溶液中的浸出化学动力学可分为化学反应控制、扩散控制和混合控制。
当浸出反应过程为液膜层扩散控制时,其动力学方程可表示为:
 (2)
(2)
当浸出反应过程为固相层扩散控制时,其动力学方程可表示为:
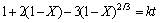 (3)
(3)
当浸出反应过程为化学反应控制时,其动力学方程可表示为:
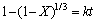 (4)
(4)
式中:X为反应物的转化率(浸出率),%;t为反应时间,min;k为表观速率常数。
为了准确描述Fe2O3在污酸中的化学动力学规律,在污酸酸度为150 g/L时,增加了一组实验温度为45 ℃下的浸出反应,结合图3中Fe2O3浸出铁浓度随时间的变化规律,将不同温度下Fe2O3在污酸中的浸出随反应时间的变化规律分为10~60 min和60~720 min两个时段进行研究。根据拟合结果发现,10~60 min的浸出效果与式(3)相关性较显著,而60~720 min的浸出率与式(4)的相关性较显著,如图6和7所示。
通过不同温度下拟合出直线斜率,得到表观速率常数k,如表3所示。
因为表观速率常数k与绝对温度的关系服从Arrhenius公式:
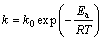 (5)
(5)
所以对上式进行两边取对数,得:
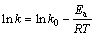 (6)
(6)
式中:k0为频率因子;Ea为表观活化能,kJ/mol;R为摩尔气体常数;T为反应温度,K。
根据表2数据,将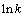 和1/T进行拟合,得
和1/T进行拟合,得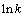 与1/T的关系曲线,如图8所示。
与1/T的关系曲线,如图8所示。
根据曲线截距和斜率,通过式(6)计算得到Fe2O3在污酸中浸出反应的表观活化能Ea和频率因子k0,具体计算结果如下表4所示。

图6 不同温度下1~60 min时段固相层扩散控制过程拟合
Fig. 6 Fitting of diffusion in solid phase layer at different temperatures in period of 10-60 min
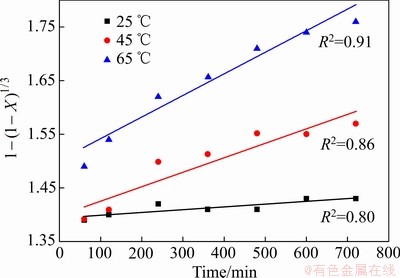
图7 不同温度下60~720 min时段化学反应控制过程拟合
Fig. 7 Fitting of chemical reaction control at different temperatures in period of 60-720 min
表3 不同温度下污酸的k值
Table 3 k values of waste acid at different temperatures
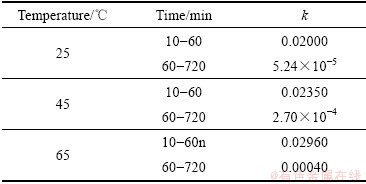
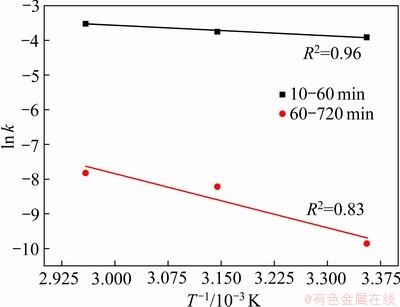
图8 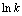 与1/T的线性拟合
与1/T的线性拟合
Fig. 8 Fitting of 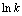 and 1/T
and 1/T
表4 反应表观活化能Ea和频率因子k0
Table 4 Apparent activation energy Ea and frequency factor k0
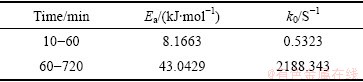
综上所述,通过计算和拟合,在25~65 ℃范围内,Fe2O3在污酸中的浸出反应在10~60 min受固相层扩散控制明显,反应表观活化能为8.1663 kJ/mol,通常这类反应的速度比较快;在60~720 min受化学反应过程控制明显,反应表观活化能为43.0429 kJ/mol,即Fe2O3在污酸中的浸出动力学模型为:
在10~60 min内,
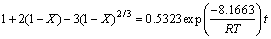 (7)
(7)
在60~720 min内,
 (8)
(8)
2.3 三氧化二铁对污酸中砷的处理研究
砷是铜冶炼污酸中存在最多的污染物,本工作模拟了Fe2O3对污酸中砷的处理情况。由图9可以看出,在反应后调节溶液pH至4~5时,各酸度下污酸中Fe2O3对砷都具有一定的去除效果。随着酸度的升高,砷的去除效果越好,最高可达95%。这是因为随污酸酸度增加,溶液中铁浓度增加,而在pH为4~5时,溶液中铁离子会形成絮凝体,吸附污酸中砷。另外,在此条件下,溶液中As5+和Fe3+会发生化学反应生成无定形砷酸铁,使砷元素进入固相,达到去除砷的效果[32]。由图10看出,砷的去除效率和铁回收效率基本保持一致,溶液中铁离子大多都回收至固相中,且污酸酸度越高,铁的回收效果越好,最高可达97%左右。

图9 不同酸度污酸中砷去除效率
Fig. 9 Arsenic removal efficiency in waste acid with different acidities

图10 不同酸度污酸中铁回收效率
Fig. 10 Iron recovery efficiency in waste acid with different acidities
3 结论
1) Fe2O3在污酸中浸出铁,当温度为65 ℃,搅拌速度为200 r/min,酸度为180 g/L,浸出时间为600 min时,可达到较好的浸出效果,且污酸溶液pH并无明显变化,多次浸出可提高Fe2O3的浸出效率。
2) Fe2O3在污酸中的反应过程可以用收缩未反应核模型描述,10~60min污酸浸出Fe2O3过程受固相层扩散控制比较明显,反应表观活化能为8.1663 kJ/mol,而60~720 min受化学反应过程控制比较明显,反应表观活化能为43.0429 kJ/mol。
3) Fe2O3可以用于处理污酸中的砷,通过调节pH至4~5,随着酸度的升高,反应浸出的铁较多,砷的处理效果越好,溶液中铁的回收效率越高,除砷效率可达95%。
REFERENCES
[1] TAMASI G, CINI R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena[J]. Science of the Total Environment, 2004, 327(1): 41-51.
[2] 郭恒萍, 高俊发. 冶炼含砷污酸与酸性含砷废水处理试验及应用研究[D]. 西安: 长安大学, 2010.
GUO Heng-ping, GAO Jun-fa. Study on the treatment and application of arsenic-containing sulphuric acid and acid arsenic-containing wastewater[D]. Xi’an: Chang’an University, 2010.
[3] 余 勇. 铅锌冶炼厂硫酸污水的处理工艺探讨[J]. 湖南有色金属, 2001, 17(4): 39-40.
YU Yong. Discussion on treatment process of sulfuric acid sewage in lead and zinc smelter[J]. Hunan Nonferrous Metals, 2001, 17(4): 39-40.
[4] SHEORAN A, SHEORAN V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review[J]. Minerals Engineering, 2006, 19(2): 105-116.
[5] 周建民, 党 志, 蔡美芳, 司徒粤, 刘丛强. 大宝山矿区污染水体中重金属的形态分布及迁移转化[J]. 环境科学研究, 2005, 18(3): 5-10.
ZHOU Jian-min, DANG Zhi, CAI Mei-fang, SITU Yue, LIU Cong-qiang. Form distribution and migration and transformation of heavy metals in polluted waters in Dabaoshan mining area[J]. Research of Environmental Sciences,2005, 18(3): 5-10.
[6] CHOONG T S Y, CHUAH T G, ROBIAH Y, KOAY F L G, AZNI I. Arsenic toxicity, health hazards and removal techniques from water: An overview[J]. Desalination, 2007, 217(1): 139-166.
[7] 应国民, 李庆超, 阴树标, 陈 雯. 铜冶炼污酸处理技术现状及发展趋势[J]. 矿冶, 2016(4): 68-72.
YING Guo-min, LI Qing-chao, YIN Shu-biao, CHEN Wen. Current situation and development trend of waste acid treatment technology in copper smelting[J]. Mining and Metallurgy, 2016(4): 68-72.
[8] 罗永光, 邹景田, 李 静. 从污酸中去除砷、氯的技术研究及进展[J]. 矿冶, 2018, 7(2): 90-95.
LUO Yong-guang, ZOU Jing-tian, LI Jing. Research and development of removal of arsenic and chlorine from waste acid[J]. Mining and Metallurgy, 2018, 7(2): 90-95.
[9] ZHENG Ya-jie, ZHANG Sheng-hua, GONG Chang. Novel technique for recovery of copper and arsenic from arsenic-containing waste acid[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 2985-2992.
[10] 曾辉平, 赵运新, 吕育锋, 李 冬, 张 杰. 水厂反冲洗铁锰泥热处理产物结构及除砷变化[J]. 中国环境科学, 2017, 37(8): 2986-2993.
ZENG Hui-ping, ZHAO Yun-xin, Lü Yu-feng, LI Dong, ZHANG Jie. Asorption behaviors towards As and structural change of iron and manganese oxide sludge by thermal treatment[J]. China Environmental Science, 2017, 37(8): 2986-2993.
[11] 王翠翠, 陈秋玲, 刘树根, 何文豪, 宁 平, 郜华萍. 超声铁盐改性黄磷水淬渣的制备及除砷性能研究[J]. 中国环境科学, 2017, 37(6): 2187-2193.
WANG Cui-cui, CHEN Qiu-ling, LIU Shu-gen, HE Wen-hao, NING Ping, GAO Hua-ping. Research on the preparation of ultrasonic-assisted ferric salts modified phosphorous slag and its As(Ⅲ) removing capacity[J]. China Environmental Science, 2017, 37(6): 2187-2193.
[12] YU Xing-wen, WEI Yuan-feng, LIU Cheng-bin, MA Jian-hong, LIU Hui, WEI Shu-dan, DENG Wei, XIANG Jian-nan, LUO Sheng-lian. Ultrafast and deep removal of arsenic in high-concentration wastewater: A superior bulk adsorbent of porous Fe2O3 nanocubes-impregnated graphene aerogel[J].Chemosphere,2019, 222: 258-266.
[13] HANSEN H K, GUTIERREZ C, FERREIRO J, ROJO A. Batch electro dialytic treatment of copper smelter wastewater[J]. Minerals Engineering, 2015, 74: 60-63.
[14] 刘 丰. 铜冶炼污酸处理技术现状及发展趋势[J]. 世界有色金属, 2018(1): 27, 29.
LIU Feng. Current status and development trend of copper smelting acid treatment technology[J]. World Nonferrous Metals, 2018(1): 27, 29.
[15] 王 强, 卜锦春, 魏世强, 黄玉明. 赤铁矿对砷的吸附解吸及氧化特征[J]. 环境科学学报, 2008, 28(8): 1612-1617.
WANG Qiang, BU Jin-chun, WEI Shi-qiang, HUANG Yu-ming. Characteristics of isothermal adsorption and desorption, and oxidation of As(Ⅲ) ion on a hematite surface[J]. Journal of Environmental Science, 2008, 28(8): 1612-1617.
[16] DE MELLO J W V, GASPARON M, SILVA J. Effectiveness of arsenic co-precipitation with Fe-Al hydroxides for treatment of contaminated water[J]. Revista Brasileira de Ciência do Solo, 2018, 42: e0170261.
[17] SENANAYAKE G, DAS G. A comparative study of leaching kinetics of limonitic laterite and synthetic iron oxides in sulfuric acid containing sulfur dioxide[J]. Hydrometallurgy, 2004, 72(1): 59-72.
[18] JEONG D, KIM K, MIN D, CHOI W. Freezing-enhanced dissolution of iron oxides: Effects of inorganic acid anions[J]. Environmental Science & Technology, 2015, 49(21): 12816-12822.
[19] OLVERA-VENEGAS P N. HERNANDEZ CRUZ L E, LAPIDUS G T. Leaching of iron oxides from kaolin: Synergistic effect of citrate-thiosulfate and kinetic analysis[J]. Hydrometallurgy, 2017, 171: 16-26.
[20] 田庆华, 王 浩, 王恒利. 盐酸体系中辉锑矿的臭氧协同氧化浸出动力学[J]. 中国有色金属学报, 2018, 28(6): 1242-1249.
TIAN Qing-hua, WANG Hao, WANG Heng-li. Ozonation synergistic leaching kinetics of stibnite in system of hydrochloric acid[J]. Journal of Chinese Nonferrous Metals, 2018, 28(6): 1242-1249.
[21] 徐志峰, 朱辉, 王成彦. 富氧硫酸体系中硫化锌精矿的常压直接浸出动力学[J].中国有色金属学报, 2013, 23(12): 3440-3447.
XU Zhi-feng, WANG Hao, WANG Heng-li. Atmospheric direct leaching kinetics of zinc sulfide concentrate in oxygen-rich sulfuric acid system[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(12): 3440-3447.
[22] 李 望, 管学茂, 朱晓波. 赤泥循环酸浸提钛实验及动力学研究[J]. 稀有金属与硬质合金, 2015, 43(3): 9-12.
LI Wang, GUAN Xue-mao, ZHU Xiao-bo. Experimental and kinetic study on titanium extraction by acid leaching in red mud[J]. Rare Metals and Cemented Carbides, 2015, 43(3): 9-12.
[23] 朱晓波, 李 望, 李文中, 汤 森, 白鹏远, 管学茂. 赤泥中钇浸出行为及动力学研究[J]. 硅酸盐通报, 2016, 35(10): 3367-3372.
ZHU Xiao-bo, LI Wang, LI Wen-zhong, TANG Sen, BAI Peng-yuan. Leaching behavior and kinetics of red mud[J]. Bulletin of the Chinese Ceramic Society, 2016, 35(10): 3367-3372.
[24] 王 琪, 姜 林. 硫酸浸出赤泥中铁、铝、钛的工艺研究[J]. 矿冶工程, 2011, 31(4): 90-94.
WANG Qi, JIANG Lin. Study on the process of leaching iron, aluminum and titanium in red mud by sulfuric acid[J]. Mining and Metallurgical Engineering, 2011, 31(4): 90-94.
[25] 陈毛毛, 陈莉荣. 铝矿厂赤泥硫酸浸出动力学研究[J]. 材料导报, 2015, 29(18): 141-145.
CHEN Mao-mao, CHEN Li-rong. Study on leaching kinetics of red mud sulfuric acid in aluminum ore[J]. Materials Review, 2015, 29(18): 141-145.
[26] CASAS J M, CRISOSTOMO G, CIFUENTES L. Speciation of the Fe(Ⅱ)-Fe(Ⅲ)-H2SO4-H2O system at 25 and 50 degrees[J]. Hydrometallurgy, 2005, 80(4): 254-264.
[27] 詹 咏, 张焕焕, 疏 醒, 沈丹妮, 董 滨. 硫铁矿烧渣铁系氧化物的酸溶特性[J]. 上海理工大学学报, 2017, 39(1): 90-96.
ZHAN Yong, ZHANG Huan-huan, SHU Xing, SHEN Dan-ni, DONG Bin. Acid solubility characteristics of iron oxides from pyrite cinders[J]. Journal of University of Shanghai For Science and Technology, 2017, 39(1): 90-96.
[28] SALMIMIES R, MANNILA M, KALLAS J, HAKKINEN A. Acidic dissolution of hematite: Kinetic and thermodynamic investigations with oxalic acid[J]. International Journal of Mineral Processing, 2012, 110/111: 121-125.
[29] CORNELL R M, SCHINDLER P W. Photochemical dissolution of goethite in acid/oxalate solution[J]. Clays and Clay Minerals, 1987, 35(5): 347-352.
[30] 张晋霞, 邹 玄, 牛福生. 含锌尘泥中锌的浸出行为及动力学[J]. 中国有色金属学报, 2018, 28(8): 204-212.
ZHANG Jin-xia, ZOU Xuan, NIU Fu-sheng. Leaching behavior and kinetics of zinc in dust containing zinc[J]. Journal of Chinese Nonferrous Metals, 2018, 28(8): 204-212.
[31] RANDHAWA N S, GHARAMI K, KUMAR M. Leaching kinetics of spent nickel-cadmium battery in sulphuric acid[J]. Hydrometallurgy, 2016, 165(1): 191-198.
[32] LIU R, YANG Z, HE Z, WU L, HU C, WU W, QU J. Treatment of strongly acidic wastewater with high arsenic concentrations by ferrous sulfide (FeS): Inhibitive effects of S(0)-enriched surfaces[J]. Chemical Engineering Journal, 2016, 304: 986-992.
Leaching behavior and leaching kinetics of ferric oxide in copper-smelting waste acid
GAO Yi-qiang, ZHAO Yi-ping, ZHANG Li-xiang, HUANG Jian-hong, TIAN Sen-lin
(Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, China)
Abstract: By studying the leaching of ferric oxide in copper-smelting waste acid at different temperatures, the leaching performance of different-acidity waste acids in 720 min and the pH value of the reaction process were investigated, and the kinetics during the reaction and the treatment effect on arsenic in waste acid were discussed. The results show that there is no significant change in the pH of the solution in the whole reaction of 720 min. The leaching reaction of ferric oxide in the copper-smelting waste acid can be described by the unreacted shrinking core model. For the reaction of ferric oxide leaching in the period of 10-60 min, the process is controlled by the diffusion in the solid phase layer, the apparent activation energy is 8.1633 kJ/mol, and the chemical reaction is obviously when the rate-controlling step in the period of 60-720 min, the apparent activation energy is 43.0429 kJ/mol. At the same time, the arsenic treatment efficiency with ferric oxide in the copper-smelting waste acid can reach 90% at 65 ℃, and thus has an effective arsenic removal effect.
Key words: ferric oxide; copper-smelting waste acid; arsenic; leaching kinetics
Foundation item: Project(2018YFC1802603) supported by the National Key Research and Development Program of China
Received date: 2019-12-13; Accepted date: 2020-04-12
Corresponding author: HUANG Jian-hong; Tel: +86-871-65920526; E-mail: 49069592@qq.com
(编辑 何学锋)
基金项目:国家重点研发计划资助项目(2018YFC1802603)
收稿日期:2019-12-13;修订日期:2020-04-12
通信作者:黄建洪,副教授,博士;电话:0871-65920526,E-mail:49069592@qq.com