
Function of grinding fly ash in production of aluminum foam
WANG Yong(王 永), YAO Guang-chun(姚广春), LI Bing(李 兵)
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The fly ash was added to melt to increase the melt viscosity. The main components are quartz and mullite in the fly ash, therefore it’s a kind of hardness ceramic particles. After grinding for 10 h, the spherical particles increase and new surface is produced which will improve their performance. The cell wall was observed by SEM, many fly ash particles are in the wall. These particles tend to increase the molten aluminum surface viscosity, postpone the exhaust course, sustain the liquid within the film and delay the onset of rupture. Finally, the aluminum foam with uniform cell structure can be obtained. The density is about 0.4 g/cm3, porosity is in the range of 85%-90% and the aperture is 4-7 mm.
Key words: aluminum foam; grinding fly ash; grinding function; melt-foaming
1 Introduction
When modern man builds large load-bearing structures, he uses dense solids: steel, concrete and glass. When nature does the same, she uses cellular materials: wood and bone coral. There must be good reasons for this[1]. Accepting this fact a considerable effort has been done to develop artificial cellular materials for industrial purposes. Different polymeric and ceramic foams have already found widespread applications as functional materials. Aluminum foam is very efficient in sound absorption, impact energy absorption, electromagnetic shielding and vibration damping[2].
Aluminum foam can be prepared by adding foaming agent to the bath of molten metal or aluminum alloy after properly adjusting the viscosity of the melt. For aluminum this can be done by adding metals such as Ca or Mg[3]. Usually the foaming agent is a powdered metal hydride that releases hydrogen (i.e. TiH2) when it comes into contacting with the molten metal. The foaming agent decomposes at the melting temperature of aluminum, releases gas and blows up the melt[4-5]. After cooling, the aluminum foam can be obtained.
In this work, an innovative way to foam aluminum is to add some grinding fly ash particles into melt. Provided the powder and the mixing procedure are appropriate, the manipulated melt has an enhanced viscosity and can be foamed. The function of grinding fly ash and mechanism of increasing viscosity is discussed.
2 Experimental
Firstly the fly ash was washed, and filtrated to remove impurity such as carbon granule, and then dried, after grinding for 10 h, took out to backup.
Utilized traditional melt-foaming by ZL101 Al alloy. Firstly 6.5%-7.5%Si and 0.2%-0.4%Mg (mass fraction), were added into the molten aluminum, and 2%-5% grinding fly ash was also added to adjust the viscosity. At 650-700 ℃, stirred and added 1%-2%foaming agent TiH2, after blow up the melt and heated for 3-8 min, then the particles reinforced aluminum foam was obtained.
3 Results and discussion
3.1 Function of fly ash
Fly ash contains several types of particles: spherical and spheroidal shapes, hollow particles and solid particles and irregular shapes[6].
The fly ash adopted in this experiment comes from Shenyang, whose chemical composition is shown in Table 1 and density is 2.311 3 g/cm3, 45 μm sieve residue is 14.7%. Its main chemical compositions are SiO2, Al2O3 and Fe2O3 (more than 85% in total), so the fly ash in the test belongs to ceramic particulates.
Table 1 Chemical compositions of fly ash (mass fraction, %)

Fig.1 shows that the size of particulates is relatively similar, most particulates are spherical shapes, which are advantage to the preparation of ceramic particles reinforced aluminum foam.

Fig.1 SEM image of fly ash
Fig.2 shows the XRD analysis result of fly ash. It can be found that the fly ash is made up of quartz and mullite, most of Al2O3 exists in mullite (3Al2O3·2SiO2).

Fig.2 XRD pattern of fly ash
3.2 Function of grinding
Grinding is an alternative processing method for increasing fineness of fly ash, although typical morphology of particles is substantially modified[7-8]. Most of the hollow particles and large, irregular shaped particles are crushed. Its chemical and mineralogical composition may remain unchanged. Fig.3 shows that grinding just makes hollow particles of the fly ash become fragment while solid particles in the fly ash remain unchanged. We can see that the spherical particles yield new surface that will improve their performance.
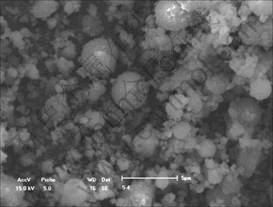
Fig.3 SEM image of fly ash after grinding for 10 h
3.3 Mechanism of wettability increase by adding fly ash
The wettability of fly ash and molten Al is not very good, the contact angles of molten Al with fly ash are larger than 90°. Improving the wettability on fly ash and molten Al has several methods[9], such as increasing temperature, adding alloy and using coating technology. Increasing temperature has some limit, it must be at a certain temperature in the test, so the way to increase temperature is infeasible. Using coating technology may change the metal/ceramic interface into metal/metal interface to improve the wettability. But the technic of coating is complex, and the coating metal reacts with parent metal to generate friable metal that will depress the material performance. Alloy addition can change the surface tension of molten aluminum and depressing surface tension can make fly ash add easily.
Fig.4 shows that part metal impact the surface tension of molten aluminum[10]. We can see that metal Mg can depress the surface tension greatly, while Si can reduce the melting point, so ZL101 aluminum alloy tends to improve the wettability between fly ash and the alloy.
Al and Mg are known to reduce SiO2 and Fe2O3 in molten aluminum alloys yield free Si and Fe, respectively. Also, Mg reduces Al2O3. At 1 000 K, using the thermodynamic data in Refs.[11], the Gibbs free energy changes associated with the possible reduction reactions taking place between ZL101 and fly ash are
SiO2+2Mg→2MgO+Si (1)
G0=-255.6 kJ
3SiO2+4Al→2Al2O3+3Si (2)
G0=-532.2 kJ
Fe2O3+2Al→Al2O3+2Fe (3)
G0=-799.6 kJ
Fe2O3+3Mg→3MgO+2Fe (4)
G0=-917.0 kJ
4Al2O3 + 3Mg → 3MgAl2O4 + 2Al (5)
G0 = -215.0 kJ
MgAl2O4 + 3Mg → 4MgO + 2Al (6)
G0 = -84.9 kJ.

Fig.4 Surface tension of aluminum alloy containing alloy element
Fig.5 shows that in-situ reactions between SiO2 in the fly ash and molten aluminum have occurred, and new dot material generates. Analyzed by EDX, these new generated dots may be Al2O3. Under a certain condition, we can prepare aluminum foam with uniform cell structure.

Fig.5 SEM image of fly ash(a) and EDX analysis result(b) of molten aluminum after in-situ reaction
Fig.6 shows the photos of cross-sectional and length-sectional aluminum foam. The density is about 0.4 g/cm3, the porosity is 85%-90% and the cell diameter is 4-7 mm.

Fig.6 Photos of cross-sectional(a) and length-sectional(b) aluminum foam
Fig.7 shows that fly ash exists in the cell wall. The ceramic particles and aluminum melt form a nonwetting material system. The high probability of the adherence of gas bubble to particles produces a decoration of the bubbles by particles.
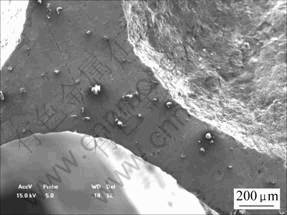
Fig.7 SEM image of cell wall
The objective is to ensure that they adhere to the gas/liquid interface within the foam, so as to stabilize the bubbles and inhibit their movement and coalescence in melt.
4 Conclusions
1) Grinding makes hollow particles of the fly ash become fragment while solid particles in the fly ash remain unchanged. The spherical particles yield new surface that improves their performance.
2) ZL101 Al alloy can depress surface tension of molten aluminum to make fly ash added more easily.
3) Fly ash and oxides which are the products of fly ash reacting with the molten aluminum enhance the surface viscosity of cell wall. So the aluminum foam can be prepared with uniform cell structure.
References
[1] BANHART J, BAUMEISTER J, WETER M. Metal foams near commercialization [J]. Met Powder Rep (MPR), 1997(4): 38-41.
[2] MAINE E M A, ASHBY M F. Applying the investment methodology for materials IMM to aluminum foams [J]. Materials & Design, 2002, 23: 307-319.
[3] BEALS J T, THOMPSON M S. Density gradient effects on aluminum foam compression behavior [J]. Journal of Materials Science, 1997, 32: 3595-3600.
[4] WANG Lu-cai, YU Li-min, WANG Fan. Development and prospect of melt-foaming process for production of metallic foams [J]. Hot Working Technology, 2004, 12: 59-63.
[5] BANHART J. Manufacture, Characterization and application of cellular metals and metal foams [J]. Progress in Materials Science, 2001, 46: 559-632.
[6] HARALD J, LENNART E, VLADIMIR R. Mechanism for performance of energetically modified cement versus corresponding blended cement [J]. Cement and Concrete Research, 2005, 35: 315-323.
[7] SUN Bao-zhen, JIA Chuan-jiu, SHUI Cui-juan. The particle morphology of pulverised fly ash (PFA) and its physical properties [J]. Journal of the Chinese Ceramic Society, 1982, 10(1): 64-76. (in Chinese)
[8] RUAN Yan, WU Ding-yan, GAO Qiong-ying. A research on particle constitution of fly ash and pozzolanic activity of fly ash ground traditionally [J]. Fly Ash Comprehensive Utilization, 2001(2): 28-30.
[9] CHEN Kang-hua, BAO Chong-xi, LIU Hong-wei. The wettability of metal ceramic (Part 2) [J]. Mater Sci Eng, 1997, 15(4): 27-34.
[10] HATCH J E. Aluminum properties and physical metallurgy [J]. ASM, 1983, 202-240.
[11] GIKUNOO E, OMOTOSO O, OGUOCHA I N A. Effect of fly ash addition on the magnesium content of casting aluminum alloy A535 [J]. Journal of Materials Science, 2004, 39: 6619-6622.
(Edited by LONG Huai-zhong)
Foundation item: Project(2002AA334060) supported by the Hi-tech Research and Development Program of China
Corresponding author: WANG Yong; Tel: +86-24-83686462; E-mail:wangyong01525@126.com