J. Cent. South Univ. (2012) 19: 918-922
DOI: 10.1007/s11771-012-1093-3
Evaluation of metal-doped manganese oxide octahedral molecular sieves in catalytic reduction of NOx from cigarette mainstream smoke
Lian Wen-liu(练文柳)1, 2, Ren Feng-lian(任凤莲)1, Liu Qi(刘琦)2, Xie Lan-ying(谢兰英)2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Research Center of China Tobacco Hunan Industrial Co., Ltd., Changsha 410014, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: A series of Ag, Cu and Co-doped manganese oxide octahedral molecular sieves (OMS-2) were synthesized and evaluated to remove nitrogen oxides (NOx) from cigarette mainstream smoke. The three kinds of catalysts were added to cigarettes for studying the capabilities of reducing NOx from cigarette mainstream smoke. The catalysis and reduction of NO in laboratory were studied. A mechanism for NOx catalytic reduction from burning cigarettes with the catalysts adding to cigarettes was described. The catalysts show excellent catalytic activity for NOx removal, especially the Ag-doped OMS-2 catalyst. 0.5% (mass fraction) Ag-doped OMS-2 catalyst has the best ability to remove NOx from cigarette mainstream smoke. The use of Ag-doped OMS-2 as catalyst for removing carcinogenic compounds from cigarette smoke will be an effective strategy to protect the environment and public health.
Key words: nitrogen oxides; catalyst; manganese oxide octahedral molecular sieve; metals; cigarette mainstream smoke
1 Introduction
Oxides of nitrogen, NOx, are produced as a result of high temperature combustion of fuels and tobacco products and are carcinogens among the Hoffmann list [1-2]. Among the oxides of nitrogen, nitric oxide, NO, and nitrogen dioxide, NO2, do harm to the health of smokers and cause pollution to the air when people smoke. NOx can cause nose and eye irritation, pulmonary edema (swelling), bronchitis, and even pneumonia in human beings. The removal of NOx from cigarette smoke becomes increasingly important. NOx removal is of practical importance for controlling the NOx poison that can come from incomplete combustion processes, e.g. cigarette combustion.
As we know, Au/TiO2 has been one of the best catalysts due to its resistance to be exposed to the atmosphere [3-4]. However, this material has some limitations in preparation processes [5]. Among the catalysts, hopcalite-type manganese oxide-based catalysts have the merits of relatively easy preparation and high activity [6-7], especially when the manganese oxide is made in the form of octahedral molecular sieves loaded with metals (OMS-2) [8]. To the present, many noble metals have been studied, such as Ag [9], Pd [10], Pt [11] and Ru [12]. Supported ceria and cerium- manganese oxide composite catalysts have been used as potential catalytic material for low temperature selective catalytic reduction (SCR) of NOx [13]. A catalyst of manganese oxide/titania materials for low temperature selective catalytic reduction was reported in removal of NOx from flue gas [14].
Cryptomelane (OMS-2) has a tunnel-structure. The tunnels consist of (2×2) matrix of edge-shared MnO6 octahedral chains that are corner shared to form a one-dimensional tunnel structure. Manganese oxides have shown promising catalytic properties in total oxidation of CO and volatile organic compounds (VOC), selective oxidation of alcohols and alkanes, as well as decomposition of H2O2, O3 and NOx [15].
In this work, a series of Ag, Cu, and Co-doped manganese oxide octahedral molecular sieves (OMS-2) were synthesized and evaluated to remove NOx from cigarette mainstream smoke. Removal levels of NOx in laboratory and from cigarette mainstream smoke were evaluated. The NOx catalytic reduction mechanism on burning cigarettes with the catalysts added to cigarettes was described.
2 Experimental
2.1 Catalyst preparation and characterization
All chemicals were of reagent grade. The catalysts of Ag-doped manganese oxide octahedral molecular sieves were synthesized as described in our previous work and other literature [15-16]. The Cu-doped OMS-2 and Co-doped OMS-2 catalysts were synthesized according to the method of Ag-doped OMS-2.
Before measurement, the catalysts were degassed at 573 K for 15 h. N2 adsorption/desorption experiments at liquid nitrogen temperature for surface area and pore size distribution (PSD) measurements were performed on a high resolution gas adsorption manometry system further equipped with a 13.33 Pa range type 120 Baratron (MKS Corporation, USA) for increasing precision at very low pressures [17]. TEM observations were performed with a JEOL JEM-2010 electron microscope. X-ray powder diffraction (XRD) patterns were recorded with a D/Max-2500/PC powder diffractometer (Rigaku, Japan) operated at 40 kV and 250 mA, using nickel filtered Cu Kα (λ=0.154 18 nm) radiation.
2.2 Cigarette preparation modified with catalysts
The normal and test cigarettes were obtained from Changde Cigarette Factory, China. The test cigarettes were modified by adding a catalyst suspension into the cut tobacco of the normal cigarettes (10 mg of Cu incorporated catalyst/cigarette, 10 mg of Co incorporated catalyst/cigarette and 10 mg of Ag incorporated catalyst/ cigarette, respectively).
Prior to analysis, the cigarettes were conditioned at (22±1) °C and at (60±2)% relative humidity for at least 48 h according to the ISO standard 3402 (ISO, 1999e).
2.3 Evaluation of catalysts for removing NOx in laboratory
Vapor phase removal of NOx was performed in a reactor, as shown in Fig. 1. As the level of NO in cigarette mainstream smoke is about 0.5 μg per cigarette, while that of CO is about 10-12 mg per cigarette, the ratios of w(CO)/w(NO) were 0, 2, and 10, respectively. The reactor temperature was 200-600 °C, as the same as the temperature of zones of tobacco pyrolysis when a cigarette is in combustion.
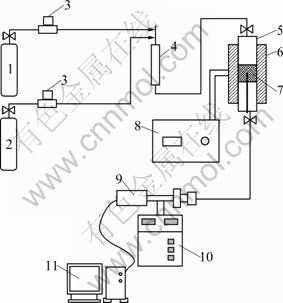
Fig. 1 Experimental setup: 1—10% (volume fraction) CO/Ar; 2—5% NO/Ar; 3—Valve; 4—Chamber of pulse mixing; 5— Reactor; 6—Heating oven; 7—Catalysts; 8—Temperature controller; 9—Mass spectrometer; 10—Control center of vacuum; 11—Computer
2.4 Evaluation of NOx removal from cigarette mainstream smoke with catalysts
After the test, cigarettes were prepared and conditioned for 48 h, and NOx levels from the normal cigarettes and the test cigarettes were determined according to our previous work reported in Ref. [18]. The smoking machine was equipped with an oxidizing tube and two absorbing bottles in series. Nitrogen monoxide in mainstream cigarette smoke was converted to nitrogen dioxide in the oxidizing tube, and was absorbed by 5% triethanolamine in a series of absorbing bottles. Then, 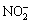 and
and 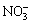 contents in the absorbing solution were determined by ion chromatography simultaneously. The results were expressed as NOx (μmol/cigarette).
contents in the absorbing solution were determined by ion chromatography simultaneously. The results were expressed as NOx (μmol/cigarette).
3 Results and discussion
3.1 Characterization of catalysts
Figure 2 shows the typical TEM pictures of Ag incorporated OMS-2 catalysts. As reported in our previous work [19], the TEM images clearly show that they are needle-like nanorods. Figure 3 shows a typical XRD pattern of the synthesized Ag/OMS-2 catalysts. There are clear main diffraction peaks at 2θ=12.16°, 17.19°, 28.17°, 37.15°, 41.19°, 49.19° and 60.11°, which show the typical structure of cryptomelane (KMn8O16, JCPDS 29-1020), that is, the structure of OMS-2 [8].
An isotherm plot for N2 adsorption/desorption shows steep increases in the low relative pressure range, which indicates the presence of micropores (shown in Fig. 4). Figure 4 also shows a typical PSD with sharp profile, which suggests the existence of a well-defined micropore structure. More details of the characterization of the Ag/OMS-2 materials had been reported elsewhere [19].
3.2 Catalysis and reduction of NOx in laboratory
NO accounts for 90% in NOx, so the removal of NOx is mainly to remove NO. The catalytic reduction of NOx on the catalysts was studied in a reactor shown in Fig. 1 with input pulses of CO/Ar and NO/Ar. The reaction of removing NO is as follows:
2NO+2CO=CO2+N2 (1)
The four catalysts 0.1% Ag/OMS-2, 0.5% Ag/ OMS-2, 0.5% Cu/OMS-2, and 0.5% Co/OMS-2 were selected to perform the catalytic reduction of NO. Figure 5 depicts the temperature dependence of the conversion rate of the four catalysts for the catalytic reduction of NO. As can be seen from Fig. 5, NO is hard to decompose below 600 °C. But the situation changes when CO is added as a reductant and NO is completely removed by the four catalysts under this condition.
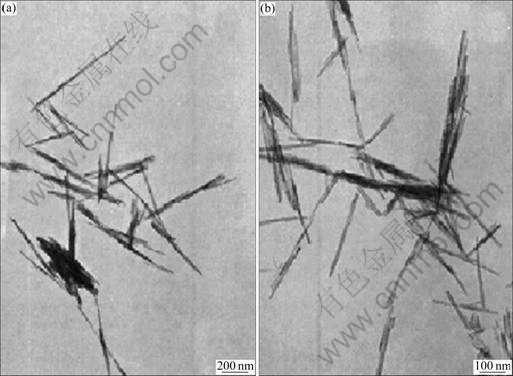
Fig. 2 TEM images of OMS-2 (a) and 0.1 Ag-doped OMS-2 catalyst (b)
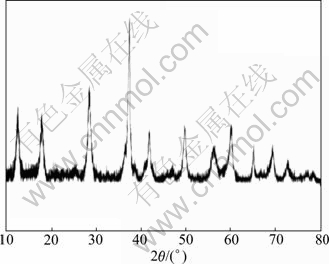
Fig. 3 Typical XRD pattern of 0.1% Ag/OMS-2
3.3 Evaluation of catalysts added to cigarettes for NOx removal
The temperature distribution in the combustion coal of a cigarette includes three parts with the direction of cigarette burning. The highest temperatures occur on the central axis of the coal (about 700-950 °C during a puff), which is the burning zone; the second part is the pyrolysis zone with temperature ranging from 200 to 600 °C; the third part is the cooling zone with temperature from room temperature to 200 °C. Figure 6 shows a content curve of oxygen with the burning axis of a cigarette in the process of every puff. Firstly, oxygen is
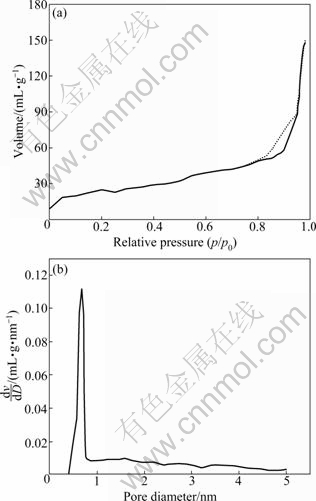
Fig. 4 N2 adsorption/desorption isotherm (a) and pore size distribution (b) of 0.1% Ag/OMS-2 nearly completely burned out with level less than 0.1% in the burning zone. The abundant CO is as a reductant to catalyze NO to N2 in the present of catalysts according to Eq. (1) as described above. Secondly, in the pyrolysis zone, the oxygen level increases to 3% due to the air dilution, and the catalysts simultaneously catalyze CO and NO into CO2 and N2.
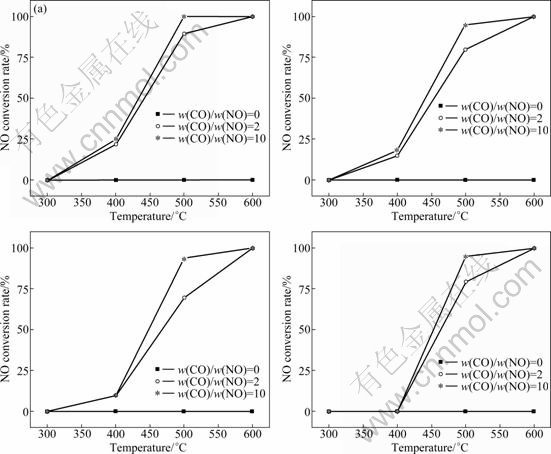
Fig. 5 NO conversion rates of different catalysts with temperature: (a) 0.5% Ag/OMS-2; (b) 0.5% Cu/OMS-2; (c) 0.1% Ag/OMS-2; (d) 0.5% Co/OMS-2
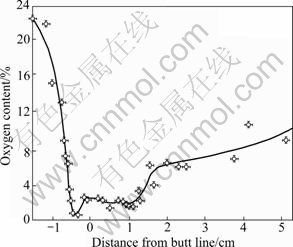
Fig. 6 Contents of oxygen in burning cigarette with direction of burning
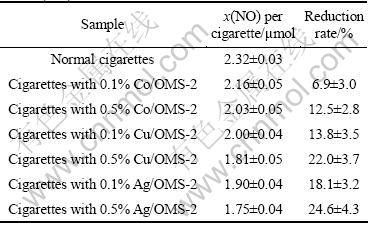
The reduction rates of NOx in cigarette mainstream smoke from the test cigarettes for different catalysts are listed in Table 1. NOx is effectively reduced up to over 20% by modified cigarettes with Ag or Cu-doped OMS-2 compared to the levels measured in the smoke from normal cigarettes. As shown in Table 1, Ag doped manganese oxide octahedral molecular sieve is better than Cu or Co-doped ones in reduction of NOx. The content of Ag in the manganese oxide octahedral molecular sieve also influences the reduction rate. Compared with 0.1% Ag/OMS-2, 0.5% Ag/OMS-2 has better ability to remove NOx from cigarette mainstream smoke.
Table 1 Reduction rates of NOx from cigarette mainstream smoke (n=5)
4 Conclusions
1) The Ag and Cu incorporated manganese oxide octahedral molecular sieves show excellent NOx catalytic activity in laboratory and in cigarettes. Among the catalysts, Ag-doped OMS-2 has better properties to remove NOx from cigarette mainstream smoke.
2) Since NOx is one of the main carcinogens in tobacco smoke, its reduction in inhaled smoke should reduce the effects of the compounds. The prepared noble metals incorporated OMS-2 catalysts may be used as promising catalysts for complete catalysis of nitrogen oxides.
Acknowledgements
This work is supported by the fund of China Tobacco Hunan Industrial Co., Ltd. The authors thank for the outstanding technical assistance from Dr. HU (HU Rong-rong, Department of Chemical Engineering of Tsinghua University, Bejing, China).
References
[1] HARIRI M H. Nitrogen oxides as atmospheric pollutants: NOx removal in the presence of oxygen with metal oxides [J]. Scientia Iranica, 1994, 1(3): 257-266.
[2] International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans [R]. Lyon, FR: IARC, 2004. 53-119.
[3] YAN Z, CHINTA S, MOHAMED A A, FACKLER J P, GOODMAN D W. CO oxidation over Au/TiO2 prepared from metal-organic gold complexes [J]. Catalysis Letters, 2006, 111(1/2): 15-18.
[4] WALTHER G, JONES G, JENSEN S, QUAADE U, HORCH S. Oxidation of methane on nanoparticulate Au/TiO2 at low temperature: A combined microreactor and DFT study [J]. Catalysis Today, 2009, 142(1/2): 24-29.
[5] ROLISON D R. Catalytic nanoarchitectures—The importance of nothing and the unimportance of periodicity [J]. Science, 2003, 299(5613): 1698-1701.
[6] KANUNGO S B. Physicochemical properties of MnO2 and MnO2—CuO and their relationship with the catalytic activity for H2O2 decomposition and CO oxidation [J]. J Catal, 1979, 58(3): 419-435.
[7] PUCKHABER L S, CHEUNG H, COCKE D L, CLEARFIELD A. Reactivity of copper manganese oxides [J]. Solid State Ionics, 1989, 32/33: 206-213.
[8] XIA G G, YIN Y G, WILLIS W S, WANG J Y, SUIB S L. Efficient stable catalysts for low temperature carbon monoxide oxidation [J]. J Catal, 1999, 185(1): 91-105.
[9] TAYLOR S H, RHODES C. The oxidation of carbon monoxide at ambient temperature over mixed copper-silver oxide catalysts [J]. Catalysis Today, 2006, 114(4): 357-361.
[10] WOOTSCH A, DESCORME C, ROUSSELET S, DUPREZ D, TEMPLIER C. Carbon monoxide oxidation over well-defined Pt/ZrO2 model catalysts: Bridging the material gap [J]. Appl Sur Sci, 2006, 253(3): 1310-1322.
[11] ALEXANDER J D, CHRISTINE A L. Abatement of CO from relatively simple and complex mixtures: III. Oxidation on Pd-CeO2/C and CeO2/C catalysts [J]. Appl Catal B: Environ, 2006, 67(1/2): 52-59.
[12] BOUCHER A C, RHUN V LE, HAHN F, ALONSO-VANTE N. The CO-adsorbate electrooxidation on ruthenium cluster-like materials [J]. J Electroanalytical Chem, 2003, 554/555: 379-384.
[13] QI G, YANG R T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx-CeO2 catalyst [J]. J Catal, 2003, 217(2): 434-441.
[14] LEI J, SREEKANTH P M, SMIRNIOTIS P G, THIEL S W, PINTO N G. Manganese oxide/titania materials for removal of NOx and elemental mercury from flue gas [J]. Energ Fuel, 2008, 22(4): 2299- 2306.
[15] JOTHIRAMALINGAM R, VISWANATHAN B, VARADARAJAN T K. Preparation, characterization and catalytic properties of cerium incorporated porous manganese oxide OMS-2 catalysts [J]. Catalysis Communications, 2005, 6: 41-45.
[16] HU Rong-rong, XIE Lan-ying, DING Shi, HOU Jian, CHENG Yi, WANG De-zheng. CO oxidation and oxygen-assisted CO adsorption/desorption on Ag/MnOx catalysts [J]. Catalysis Today, 2008, 131: 513-519.
[17] LI Wen-jun, WANG Yao, WANG De-zheng, WEI Fei. An adsorption manometry apparatus for high resolution and low pressure adsorption isotherms [J]. Chin J Catal, 2006, 27(3): 200-202. (in Chinese)
[18] PENG Hui-ming, WU Ming-jian, HE Zhi-hui, LIAN Wen-liu. Determination of nitrogen oxides in cigarette mainstream smoke by ion chromatography [J]. Chinese Journal of Anal Chem, 2007, 35(2): 289-292. (in Chinese)
[19] HU Rong-rong, CHENG Yi, XIE Lan-ying, WANG De-zheng. Effect of doped Ag on performance of manganese oxide octahedral molecular sieve for CO oxidation [J]. Chin J Catal, 2007, 28(5): 463-468. (in Chinese)
(Edited by HE Yun-bin)
Received date: 2011-02-23; Accepted date: 2011-07-21
Corresponding author: Xie Lan-ying, PhD, Tel: +86-15802696067, E-mail: xielanying@baisha.com