J. Cent. South Univ. (2016) 23: 2191-2198
DOI: 10.1007/s11771-016-3276-9

Desilication kinetics of calcined boron mud in molten sodium hydroxide media
NING Zhi-qiang(宁志强), SONG Qiu-shi(宋秋实), ZHAI Yu-chun(翟玉春), XIE Hong-wei(谢宏伟), YU Kai(于凯)
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract: Desilication kinetics of calcined boron mud (CBM) occurring in molten sodium hydroxide media was investigated. The effects of factors such as reaction temperature and NaOH-to-CBM mass ratio on silicon extraction efficiency were studied. The results show that silicon extraction efficiency increases with increasing the reaction time and NaOH-to-CBM mass ratio. There are two stages for the desilication process of the calcined boron mud. The overall desilication process follows the shrinking-core model, and the first and second stages of the process were determined to obey the shrinking-core model for surface chemical reaction and the diffusion through the product layer, respectively. The activation energies of the first and second stages were calculated to be 44.78 kJ/mol and 15.94 kJ/mol, respectively.
Key words: boron mud; sodium hydroxide; silicon dioxide; kinetics; desilication
1 Introduction
China ranks third in the world, behind the United States and Turkey, in boron reserves and most of these boron resources are in the form of boromagnesite (Mg2B2O5·H2O) and ludwigite ((Mg,Fe)2Fe([BO3]O2) ore (B2O3 8%-12%)[1-4]. The boron resources of the United States are mainly borate minerals [1, 5-6] (B2O3 25%-50%). Turkey’s resources are associated with borax in the mineral tincal (Na2B4O7·10H2O) [1, 6-8] as well as other boron resources such as colemanite Ca[B3O4(OH)3]·H2O), ulexite (NaCa[B5O6(OH)6]·5H2O), inyoite (Ca[B3O3(OH)5]·4H2O) and hydroboracite (CaMg[B3O4(OH)3]·3H2O [6, 9-14] (B2O3 26%-42%). Therefore, it is easy to produce borax or boric acid by simply processing colemanite, ulexite, inyoite and hydroboracite because of their high boron contents and little impurity. However, China’s boron resources of boromagnesite and ludwigite ore have a low boron content with multielement impurities such as Mg, Fe and Al [15-16]. Alkaline leaching of boron from boromagnesite or ludwigite ore is the main viable option for processing. Alkaline leaching of these minerals produces a solid waste called boron mud (BM). Four tons of boron mud will be produced for every ton of borax produced. The unprocessed boron mud occupies a large area and causes a serious soil pollution problem due to its strong basicity. Boron mud production has reached 17 million tons in Liaoning Province, China. Thus, it is an urgent and challenging task to effectively utilize the boron mud.
The specific composition of boron mud depends on the properties of the raw materials and the conditions under which it is produced,although it generally consists of magnesia and silicon oxide.
At present, many researchers who investigate boron mud processing focus on leaching magnesia from boron mud to produce magnesia products such as magnesium sulfate, magnesium carbonate, magnesium hydroxide, magnesium oxide [15-21]. Few researchers have studied silicon oxide removal from boron mud in molten sodium hydroxide media.
In this work, one new technology was established for silicon oxide removal from calcined boron mud in molten sodium hydroxide media [22]. The process creates the conditions for high value-added utilization of boron mud. The objective of this work is to assess the effects of different variables, such as reaction time and NaOH-to-CBM mass ratio, on silicon extraction efficiency, to optimize the conditions and determine the desilication kinetics of calcined boron mud in molten sodium hydroxide media.
2 Experimental
2.1 Materials
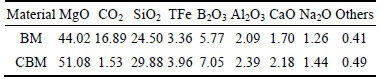
The boron mud and calcined boron mud that were used in all the experiments were obtained from Liaoning province in China. They were examined by ICP-OES, and the chemical analysis results are presented in Table 1.
Table 1 Chemical composition of BM and CBM (mass fraction, %)
The X-ray diffraction (XRD) patterns of the boron mud and the calcined boron mud (700 °C for 4 h) shown in Fig. 1 indicate that magnesium carbonate (MgCO3), magnesium orthosilicate (Mg2SiO4) and its heterogeneous isomorphism with Fe instead of Mg are the major phases in the boron mud. Magnesium carbonate decomposes into magnesium oxide and carbon dioxide at high temperature, so mass content of magnesium borate and silicon dioxide increased in the CBM because of the loss of carbon dioxide. Therefore, they can be detected by XRD in the CBM.
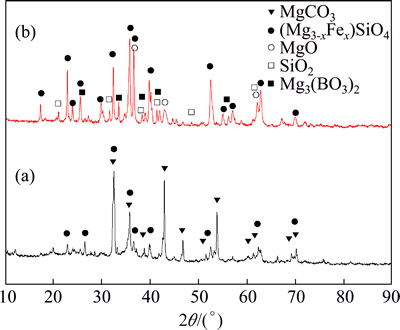
Fig. 1 XRD patterns of BM (a) and CBM (b)
2.2 Method
Specified mixtures of the CBM and sodium hydroxide were placed into stainless steel crucibles. The ratio of NaOH-to-CBM ranged from 1.0 to 1.75 in interval of 0.25. A resistance heated furnace was used. The temperature of the furnace was controlled by a programmable temperature controller with a precision of ±2 °C. When the temperature reached the set value, the stainless steel crucible with the mixture was placed into the resistance furnace. After thermal exposure in the furnace for the desired time, the sample was rapidly quenched in cool water to cease the reaction, then the sample was leached with water at 90 °C for 40 min using a stirring speed of 450 r/min. After leaching, the dehydrated solid residual was obtained after dehydrating the solid residual at 400 °C for 4 h. The content of silicon in sodium silicate solution was determined using chemical titration [23], and the silicon dioxide extraction efficiency is calculated as
 (1)
(1)
where α(SiO2) is the silicon dioxide extraction efficiency; m'(SiO2) is the mass of silicon dioxide in sodium silicate solution; m(SiO2) is the mass of silicon dioxide in the BM ores.
3 Results and discussion
3.1 Chemical reaction
The main chemical reactions occuring between the CBM and NaOH at high reaction temperature are as follows:
Mg2SiO4(s)+2NaOH(l)=Na2MgSiO4(s)+MgO(s)+ 2H2O(g)↑ (2)
SiO2(s)+4NaOH(l)=Na4SiO4(s)+2H2O(g)↑ (3)
2Mg3(BO3)2(s)+2NaOH(l)=Na2B4O7(s)+6MgO(s)+ H2O(g)↑ (4)
The main chemical reactions occurring during the leaching process at 90 °C for 40 min using a stirring speed of 450 r/min are as follows:
Na2MgSiO4(s)+2NaOH(aq)=Na4SiO4(aq)+Mg(OH)2(s)+ H2O(l) (5)
The main chemical reaction occurring at 400 °C for drying the solid residual is as follows:
Mg(OH)2(s)=MgO(s)+H2O(g)↑ (6)
3.2 Effect of reaction temperature
The influence of reaction temperature on the silicon dioxide extraction efficiency with the NaOH-to-CBM mass ratio 1.75:1 is shown in Fig. 2
The temperature has a noticeable influence on the extraction efficiency of silicon dioxide. The extraction efficiency of silicon dioxide increases with an increase in reaction temperature. Figure 2 also shows that the effect of reaction temperature on SiO2 extraction efficiency is divided into two parts: 0-20 min is the first stage with rapid extraction rate; 20-50 min is the second stage with slower extraction rate.

Fig. 2 Effect of reaction temperature on SiO2 extraction efficiency
As the temperature increases from 400 °C to 550 °C, the extraction efficiency of SiO2 increases from 34.60% to 91.90% at the reaction time of 20 min, and it increases from 43.89% to 93.88 % at the reaction time of 50 min. At 400 °C, the increment of SiO2 extraction efficiency is 9.29% (43.89%-34.60%) for the reaction time from 20 min to 50 min; while, at 550 °C, it is only 1.98% (93.88%-91.90%) for the same reaction time. The reason is that silicon dioxide in CBM tended to react completely at 550 °C for 20 min. Therefore, the extraction efficiency of SiO2 increased a little after 20 min at 550 °C. Higher temperature is not necessary due to the higher energy costs associated with the process.
3.3 Effect of NaOH-to-CBM mass ratio
The influence of the NaOH-to-CBM mass ratio on the extraction efficiency of silicon dioxide at 550 °C is shown in Fig. 3.
As shown in Fig. 3, the silicon dioxide extraction efficiency was improved with increasing the NaOH-to-CBM mass ratio. Excess sodium hydroxide is necessary to facilitate full contact of sodium hydroxide with CBM and ensure sufficient reagent. Figure 3 also shows that extraction efficiency increased rapidly in the 0-20 min time range while it is slow down in the 20-50 min time range. Therefore, the effect of NaOH-to-CBM mass ratio on SiO2 extraction efficiency can be divided into two parts.
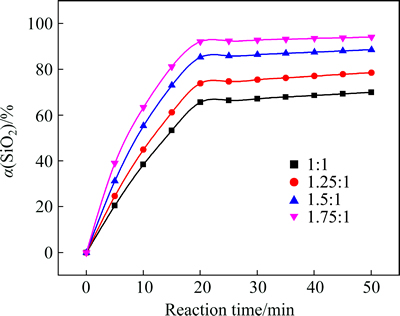
Fig. 3 Effect of NaOH-to-ore mass ratio on SiO2 extraction efficiency
3.4 Kinetics analysis
Desilication of the calcined boron mud in a molten sodium hydroxide media is typical liquid-solid reaction. The reaction between the silicon in the CBM and sodium hydroxide produces soluble sodium silicate and sodium magnesium silicate, while magnesium in the CBM transfers into magnesium hydroxide via the interaction of the molten sodium hydroxide and the generated sodium magnesium silicate at the particle surface, which has a solid product layer that can be analyzed using the shrinking-core model [24]. According to the model, the rate of reaction between solid particle and reaction reagent may be controlled by one of the following steps: diffusion through the fluid, diffusion through the product layer or the chemical reaction at the surface. It is well known that the reaction rate is strongly affected by temperature. However, this effect is smaller when the diffusion through the fluid is the rate-controlling step. Because temperature has a strong influence on the extraction efficiency of silicon dioxide, the desilication of the calcined boron mud in molten sodium hydroxide isn’t likely controlled by the diffusion through the fluid.
In order to determine the kinetic parameters and the rate-controlling step for the extraction efficiency of silicon oxide from the CBM, the following expression can be used to describe the desilication kinetics of the process if the surface chemical reaction is the rate-controlling step:
1-(1-α)1/3=krt (7)
Similarly, when the diffusion through the product layer is the rate-controlling step, the following expression for diffusion through a product layer can be used to describe the desilication kinetics.
1-3(1-α)2/3+2(1-α) = kdt (8)
The experimental data presented in Fig. 2 and Fig. 3 were fitted with Eq. (7) and Eq. (8), and the results are shown in Figs. 4-7. In Table 2, the apparent rate constants and correlation coefficient values are given for surface chemical reaction and diffusion through the product layer. The linear relationship between 1-(1-α)1/3 and the first and the second stage reaction times can be seen in Fig. 4 and Fig. 6 at varying reaction temperatures and NaOH-to-CBM mass ratios, respectively; while there is not a good linear relationship between 1-3(1-α)2/3+2(1-α) and the first stage reaction period (0-20 min) for the reaction temperature and the NaOH-to-CBM mass ratio. this model works for the second stage reaction period (20-50 min) as shown in Fig. 5 and Fig. 7.
The Arrhenius equation can be represented in modified form as [25]
lnk=lnk0-E/(RT) (9)
where k is the reaction rate constant; k0 is the pre- exponential factor; E is the reaction activation energy;R is the mole gas constant (8.314 kJ/mol); and T is the thermodynamic temperature.
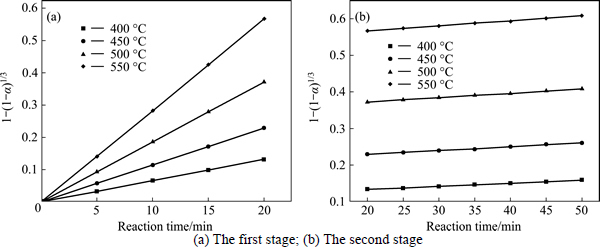
Fig. 4 Plot of 1-(1-α)1/3 vs time at different temperatures:
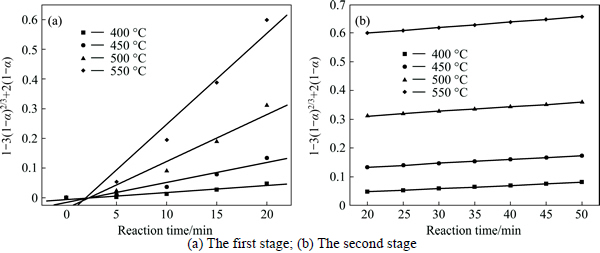
Fig. 5 Plot of 1-3(1-α)2/3+2(1-α) vs time at different temperature:
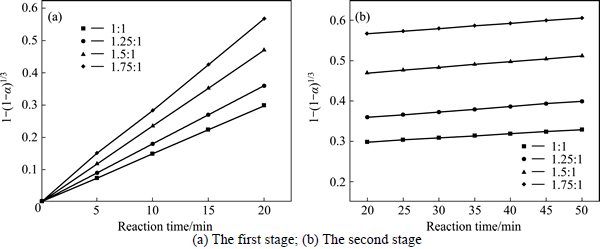
Fig. 6 Plot of 1-(1-α)1/3 vs time at different NaOH-to-CBM mass ratios:
The apparent rate constants that were determined from the straight lines in Fig. 4, Fig. 5(b), Fig. 6 and Fig. 7(b) (shown in Table 2) were used to calculate the activation energy by making a plot of ㏑k vs. T-1. The Arrhenius plots of surface chemical reaction and diffusion through the product layer are shown in Fig. 8 and Fig. 9, respectively. The activation energies are listed in Table 3.
The apparent activation energy of the first stage and second processes were calculated to be 44.78 kJ/mol, and 13.89 kJ/mol by eq. (7), respectively. The apparent activation energy of 15.94 kJ/mol was calculated by eq. (8) for diffusion through the product layer in the second stage. The apparent activation energies are usually greater than 40 kJ/mol [25-27] and between 8 and 20 kJ/mol [25] for a chemically controlled process and a diffusion controlled reaction, respectively. In accordance with these results, the first stage process is believed to obey the surface chemical reaction model. The second stage appears to be controlled by the diffusion through the product layer.
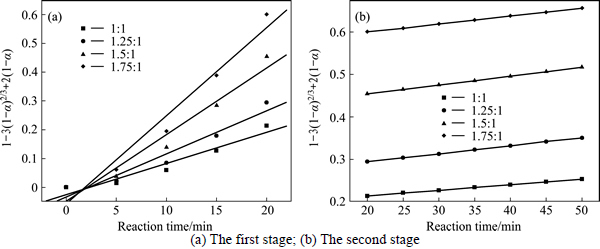
Fig. 7 Plot of 1-3(1-α)2/3+2(1-α) vs time at different NaOH-to-CBM mass ratios:
Table 2 Apparent rate constant and correlation coefficient values
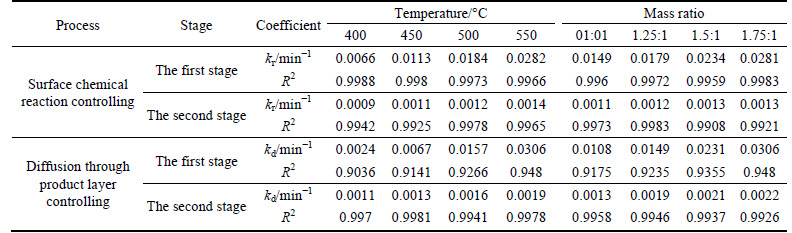
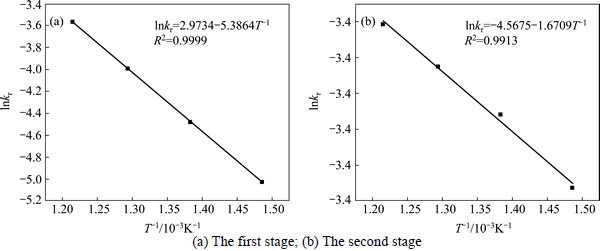
Fig. 8 Arrhenius plot of surface chemical reaction controlling:
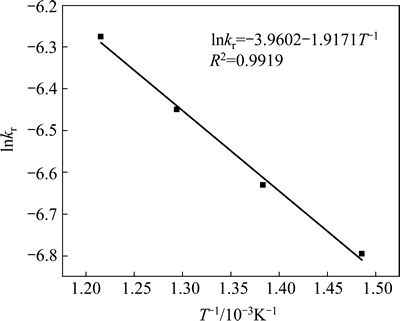
Fig. 9 Arrhenius plot of diffusion through product layer controlling at the second stage
3.5 Characterization of solid residual
The dry solid residual, obtained after reaction at 550 °C for 20 min under the conditions of NaOH-to- CBM mass ratio of 1.75:1, was characterized by SEM, SEM-EDS, XRD and chemical composition analysis.
The SEM images of the CBM and the dry solid residual are presented in Fig. 10. It can be seen that the dry solid residual particles (Fig. 10(b)) are rough and broken after desilication in the molten sodium hydroxide system in comparison to the feed material particles (Fig. 10(a)), most of whose size are less than 10 mm and homogeneous.
The EDS of the dry solid residual is presented in Fig. 11. It can be seen that there are several elements of Mg, Si, O, Na, B, Al, Fe in the dry solid residual.
Table 3 Activation energies and aqueous desilication kinetic equations of calcined boron mud
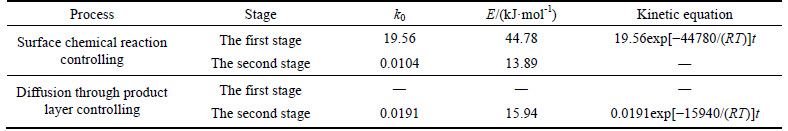
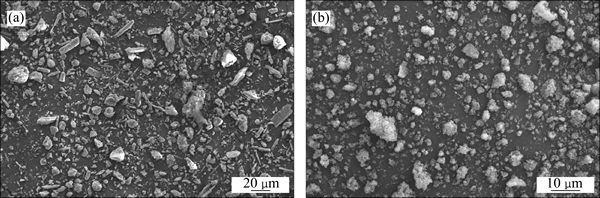
Fig. 10 SEM micrograph of CBM (a) and dry solid residual after aqueous leaching (b)
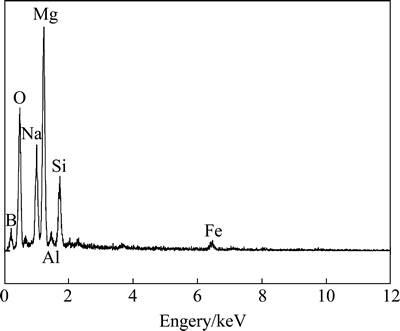
Fig. 11 EDS of dehydrated solid residual after aqueous leaching
The XRD patterns of the CBM after exposure in molten sodium hydroxide and the dry solid residual are shown in Fig. 12, where it is shown that there are Na2MgSiO4, Na4SiO4, Na4B2O7 and MgO phases in the CBM after exposure in molten sodium hydroxide and there is only MgO and a small amount of decomposed Mg(OH)2 phase in the dry solid residual, indicating that the main reactions occurring between CBM and sodium hydroxide are reactions (2), (3) and (4) at high temperature, reaction (5) occurs during the leaching process at 90 °C for 40 min. Reaction (6) occurs during the drying process at 400 °C for 4 h. The products of Na4SiO4, Na4B2O7 dissolved into the solution during this process, and therefore only MgO and a small amount of decomposed Mg(OH)2 phase are in the dehydrated solid residual, which also indicates that the SiO2 extraction efficiency is high.
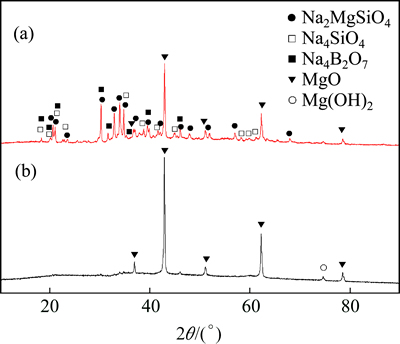
Fig. 12 XRD patterns of CBM after exposure in molten sodium hydroxide (a) and dehydrated solid residual after aqueous leaching (b)
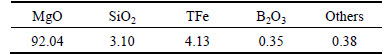
The chemical composition of dry solid residual was examined by ICP-OES, and the analytical results are shown in Table 4. Compared with Table 1, it can be seen that the content of silicon dioxide and boron oxide are reduced [28], and the contents of magnesium and iron are increased to reach a content of magnesium oxide of 92.04%.
The purpose of accumulating other elements by extracting silicon from CBM is realized and the residual solids can be used as raw materials to produce high purity magnesium products. Although, the content of iron is increased, the contents of iron and silicon dioxide are still low, and they cannot usually be accurately evaluated by XRD. Therefore, it is possible that they transferred to amorphous phase after desilication reaction based on the data shown in Figs. 11 and 12.
Table 4 Chemical composition of dehydrated solid residual after aqueous leaching (mass fraction, %)
4 Conclusions
1) The effects of reaction parameters on the desilication of calcined boron mud were examined. The results show that the extraction efficiency of silicon dioxide increases with increasing reaction temperature, reaction time and NaOH-to-CBM mass ratio.
2) The extraction efficiency of silicon dioxide is as high as 93.88% under the reaction conditions of 550 °C, 50 min and a mass ratio of NaOH-to-CBM of 1.75:1. Iron and magnesium are enriched in the residual solids, and the content of magnesium oxide is 92.04%.
3) The desilication process of calcined boron mud in molten sodium hydroxide media includes two stages. The first and second stages were determined to obey the surface chemical reaction model and the diffusion model through product layer, respectively. The activation energies of the first and second stages were calculated to be 44.78 kJ/mol and 15.94 kJ/mol, respectively. The desilication kinetics equations of the first and second stages can be expressed as 1-(1-α)1/3=19.56exp[-44780/ (RT)]t and 1-3(1-α)2/3+2(1-α)=0.0191exp[-15940/ (RT)]t.
References
[1] TANG Yao, CHEN Chun-lin, XIONG Xian-xiao, GAO Peng. World boron distribution and current status of its exploitation and development [J]. Modern Chemical Industry, 2013, 33(10): 1-4, 6. (in Chinese)
[2] LI Dong-ming. World boron resources and strategy for China’s boron development [J]. Geological Economy of China, 1989(9): 17-21. (in Chinese)
[3] JIANG Shao-yong, PALMER M R, PENG Qi-ming, YANG Jing-hong. Chemical and stable isotopic compositions of proterozoic metamorphosed evaporites and associated tourmalines from the Houxianyu borate deposit, eastern Liaoning, China [J]. Chemical Geology, 1997, 135(3/4): 189-211.
[4] PENG Qi-ming, PALMER M R. The palaeoproterozoic boron deposits in eastern Liaoning, China: A metamorphosed evaporite [J]. Precambrian Research, 1995, 72(3/4): 185-197.
[5] Tanner L H. Borate formation in a perennial lacustrine setting: Miocene-Pliocene Furnace Creek Formation, Death Valley, California, USA [J]. Sedimentary Geology, 2002, 148(1/2): 259-273.
[6] SIMONE A K, ANETTE M,  G V, RICARDI N A, GERHARD F. Boron isotope composition of geothermal fluids and borate minerals from salar deposits (central Andes/NW Argentina) [J]. Journal of South American Earth Sciences, 2004, 16(8): 685-697.
G V, RICARDI N A, GERHARD F. Boron isotope composition of geothermal fluids and borate minerals from salar deposits (central Andes/NW Argentina) [J]. Journal of South American Earth Sciences, 2004, 16(8): 685-697.
[7] CEYHAN A A, SAHIN  , BULUTCU A N. Crystallization kinetics of the borax decahydrate [J]. Journal of Crystal Growth, 2007, 300(2): 440-447.
, BULUTCU A N. Crystallization kinetics of the borax decahydrate [J]. Journal of Crystal Growth, 2007, 300(2): 440-447.
[8]  H.,
H.,  ZDEMI B. Experimental determination of the metastable zone width of borax decahydrate by ultrasonic velocity measurement [J]. Journal of Crystal Growth, 2003, 252(1/2/3): 343-349.
ZDEMI B. Experimental determination of the metastable zone width of borax decahydrate by ultrasonic velocity measurement [J]. Journal of Crystal Growth, 2003, 252(1/2/3): 343-349.
[9] BULUTCU A N, ERTEKIN C O, KUSKAY CELIKOYAN M B. Impurity control in the production of boric acid from colemanite in the presence of propionic acid [J]. Chemical Engineering and Processing, 2008, 47(12): 2270-2274.
[10] AHMET E, NIZAMETTIN D, ASIM K. Dissolution kinetics of ulexite in acetic acid solutions [J]. Chemical Engineering Research and Design, 2008, 86(9): 1011-1016.
[11] NIZAMETTIN D. Dissolution kinetics of ulexite in ammonium nitrate solutions [J]. Hydrometallurgy, 2009, 95(3/4): 198-202.
[12] TUBA D H, AHMER Y. Kinetic investigation of reaction between ulexite ore and phosphoric acid [J]. Hydrometallurgy, 2009, 96(4): 294-299.
[13] MORALES G V, CAPRETTO M E, FUENTES. L M, QUIROGA O D. Dissolution kinetics of hydroboracite in water saturated with carbon dioxide [J]. Hydrometallurgy, 58(2): 127-133.
[14]  T, CAHIT H A, MELIS S. High arsenic and boron concentrations in groundwaters related to mining activity in the Bigadic borate deposits (Western Turkey) [J]. Applied Geochemistry, 2008, 23(8): 2462-2476.
T, CAHIT H A, MELIS S. High arsenic and boron concentrations in groundwaters related to mining activity in the Bigadic borate deposits (Western Turkey) [J]. Applied Geochemistry, 2008, 23(8): 2462-2476.
[15] MA Xi, MA Hong-wen, JIANG Xiao-qian. Preparation of magnesium hydroxide nanoflowers from boron mud via anti-drop precipitation method [J]. Materials Research Bulletin, 2014, 56(8): 113-118.
[16] YIN Yu-xia, ZHANG Yi-he, ZHEN Zhi-chao. Thermal degradation and flame retarding characteristics of polypropylene composites incorporated with boron mud [J]. Composites Science and Technology, 2013, 85(8): 131-135.
[17] YANG Li-li, WANG Hong-ming, ZHU Xiang, LI Gui-rong. Effect of boron mud and CaF2 on surface tension and density of CaO-SiO2-B2O3 ternary slag systems [J]. Journal of Iron and Steel Research, International, 2014, 21(8): 745-748.
[18] NING Zhi-qiang, ZHAI Yu-chun, ZHOU Di, CAO Yong-xin, GU Hui-min. Study of the technique for the preparation of epsosmalt from the boron mud [J]. Light Metal, 2007(7): 61-63. (in Chinese)
[19] LI Ye-tao. Study on industrialization of producing light magnesium carbonate from boron mud [J]. Liaoning Chemical Industry, 2001, 30(7): 307-309. (in Chinese)
[20] YU J C, XU An-wu, ZHANG Li-zhi, SONG Rui-qi, WU Ling. Synthesis and characterization of porous magnesium hydroxide and oxide nanoplates [J]. The journal of physical chemistry B, 2004, 108(1): 64-70.
[21] WEI Zhong-qing, QI Hua, MA Pei-hua, BAO Ji-qing. A new routeto prepare magnesium oxide whisker [J]. Inorganic Chemistry Communications, 2002, 5(2): 147-149.
[22] MU Wen-ning, ZHAI Yu-chun. Desiliconization kinetics of nickeliferous laterite ores in molten sodium hydroxide system [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(2): 330-335.
[23] GB/T 4209—2008. sodium silicate for industrial use[S]. (in Chinese)
[24] LEVENSPIEL O. Chemical reaction engineering [M]. New York: John Wiley & Sons, 1999: 570.
[25] ABEL E A. Kinetics of sulfuric acid leaching of low-grade zinc silicate ore [J]. Hydrometallurgy, 2000, 55(3): 247-254.
[26] MOHAMMAD A, ZAFAR I Z, TARIQ M A. Selective leaching kinetics and upgrading of low-grade calcareous phosphate rock in succinic acid [J]. Hydrometallurgy, 2005, 80(4): 286-292.
[27] ZHAO You-cai, ZHANG Cheng-long, JIANG Jia-chao. Hydrometallurgy technology in alkaline medium [M]. Beijing: Metallurgy Industry Press, 2009. (in Chinese)
[28] NING Zhi-qiang, ZHANG Yu-chun, SONG Qiu-shi. Extracting B2O3 from calcined boron mud using molten sodium hydroxide [J]. Rare Metals, 2015, 34(10): 744-751.
(Edited by YANG Hua)
Foundation item: Project (51204037) supported by the National Natural Science Foundation of China; Project (N140204016) supported by the Fundamental Research Funds for the Central Universities, China
Received date: 2015-07-21; Accepted date: 2015-09-21
Corresponding author: NING Zhi-qiang, Lecturer, PhD; Tel: +86-24-83673860; E-mail: ningzq@smm.neu.edu.cn