Trans. Nonferrous Met. Soc. China 24(2014) 1629-1634
Electrochemical mechanism of electrolysis codeposition of Mg-Sr alloy in mol ten salt
Xiu-yun SUN, Gui-min LU, Shu-di FAN
National Engineering Research Center for Integrated Utilization of Salt Lake Resources, Key Laboratory of Safety Science of Pressurized System, Ministry of Education, East China Uni versity of Science and Technology, Shanghai 200237, China
Received 2 February 2013; accepted 20 September 2013
Abstract: The electrochemical process of Mg-Sr codeposition was studied in MgCl2-SrCl2-KCl melts containing different MgCl2 concentrations at 700 °C by cyclic voltammetry, chronopotentiometry and chronoamperometry. The results show that the actual precipitation potential of Sr reduces by nearly 0.5 V because of the depolarization effects of Sr activity reduced by forming Mg-Sr alloy. The codeposition potential condition of Mg and Sr to form Mg-Sr alloy is as follows: When electrode potential is more negative than -1.5 V, the magnesium will precipitate; when elec trode potential is more negative than -2.0 V, the magnesium and strontium will both deposit. The con trol step of codeposition process of Mg and Sr is not diffusion control step. The codeposition cur rent condition of Mg and Sr to form Mg-Sr alloy by chronoptentiometry is as follows: cathode current densities are higher than 0.71, 1.57 and 2.83 A/cm2 in MgCl2-SrCl2-KCl melts with MgCl2 concentrations of 2%, 5% and 10% (mass fraction), respectively.
Key words: molten salt electrolysis; Mg-Sr alloy; electrochemical reduction
1 Introduction
Sr can be used as a magnesium alloy modificator. Adding a small amount of Sr into magnesium alloy can refine the grain and improve its mechanical properties [1]. Mean while, Sr can also be used as an alloying element to improve the heat resistance of magne sium alloy [2]. Sr is also widely used as efficient modificators in aluminum alloy. Generally, it is added to the alloy in the form of Al-Sr master alloys. However, Mg-Sr master alloys are superior than Al-Sr mas ter alloys. Al-Sr alloy has mostly eutectic struc ture containing a lot of thick high-temperature phase Al4Sr. Its dissolved absorp tion is slow and Sr burns seriously. The melting point of Mg-Sr alloy is lower than that of Al-Sr alloy. Mg-Sr alloy does not contain high-temperature phase that is difficult to break down. The dissolved absorption of Sr is faster and Sr burns to a less extent [3]. Mg-Sr alloys and multicomponent alloys have been studied by ARGYROPOULOS and CHOW [4], and Mg-Sr alloys are used in the modification of aluminum alloy.
The main methods to produce Mg-Sr alloys are mixed method and melt-immerse reduction. The mixed method can be used in the preparation of Mg-Sr alloys, but it needs preparation of metal Mg and Sr in advance, which are then remelted and doped into alloys. Therefore, the process is long and the energy consumption is high. The melt-immersion reduction is achieved by adding Sr compounds to the Mg melt and Mn functions as a reducing agent and the Sr concentration gradi ent of the reaction interface functions as the ther modynamic and kinetic driving force. Sr precipi tated from Sr compound diffuses continu ously into the Mg melt, forming Mg-Sr alloy. However, due to the slow diffusion rate of reduction, only the Mg-Sr alloy containing a trace amount of Sr (generally not higher than 0.02%) can be prepared [5]. Molten salt electroly sis codeposition can prepare the metal which is difficult to achieve from an aqueous solution and it has a high deposition rate. Also, it makes the current efficiency higher than that by the codeposition and alloying process proceed simultaneously.
Molten salt electrolysis method has been used in the preparation of Mg-Li [6,7], Al-Ca [8], Mg-Li-La [9], Mg-Li-Gd [10] and other alloys [11-13]. As the solubility of Sr in strontium chloride molten salt reaches up to 20%, the current effi ciency of elemental Sr is lower by molten salt electrolysis [14]. Although the study of prepara tion of Mg-Sr master al loy by molten salt electrolysis is significant, the preparation of Mg-Sr master alloy by molten salt electrolysis has not been reported yet. Theoreti cal decomposition potential difference between Mg2+ and Sr2+ is nearly 1 V, from which it is gener ally believed that molten salt code- position is very difficult. But because of the formation of binary alloys, the activ ity of alloying elements can be effectively reduced [15]. It may promote the dissolution and diffusion of the alloying and has depolarization effect which makes molten salt electrolysis codeposition possible. In this work, the electrochemical mechanism of different MgCl2 concentrations of Mg-Sr alloy in electrodeposition process was investigated, and the reduction potential and current parameters of Mg2+ and Sr2+ in an inert cathode in molten salt system were identified, which provides some theo retical guidance for industrialization prepara tion of Mg-Sr alloys by molten salt electrol ysis codeposition.
2 Experimental
The electrochemical experimental apparatus used in the experiment is shown in Fig. 1. The elec trolyte melt temperature of electrochemical test as 700 °C. The working electrode was W electrode (d 1 mm) and the electrode area of W wire cath ode was controlled by the depth of the molten salt. The auxiliary electrode and reference electrode were spectral pure graphite rods (d 8 mm). In order to further remove impurities, pre-electrolysis at potential of -1.3 V (vs spectral pure graphite reference electrode) was carried out for 3 h before starting the experiment.
3 Results and discussion
3.1 Cyclic voltammetry
Figure 2 shows the typical cyclic voltamme try curves in different concentrations of magnesium chloride in MgCl2-SrCl2-KCl melts on the W electrode. According to Fig. 2, as the potential scans in the negative direction, the current starts to increase sharply at about -1.6 V. With the continuing negative shift of potential, the peak reduction current appears; when the potential continues to scan in the negative direction to about -2.1 V, the current increases sharply again.
Comparing different MgCl2 concentrations of cyclic voltammetry curves, the deposition potential of Mg and Sr has little change as MgCl2 concentration gradually increases. It demon strates that Mg2+ and Sr2+ concentration ratio in molten salt has no effect on Mg and Sr deposition potential. However, the impact is the depolarization owing to precipita tion of strontium in liquid magnesium, which results in Sr2+ increasing by 0.5 V in the actual deposi tion potential. With MgCl2 concentration gradually increases, the reduc tion peak current of the Mg2+ also increases gradually, mainly for that concentrat ion of Mg2+ is higher, the limiting diffusion current is larger in the same cathode electrolysis area.

Fig. 1 Schematic diagram for electrochemical experi ment apparatus

Fig. 2 Cyclic voltammetry curves for MgCl2-SrCl2-KCl melts containing different MgCl2 concentrations (mass fraction)
3.2 Chronoamperometry
In order to study the electrochemical behav ior of Mg-Sr alloys by Mg-Sr codeposition further, current signals with time of the 2% MgCl2-SrCl2-KCl melts on W electrode at differ ent applied potentials at 700 °C are shown in Fig. 3.

Fig. 3 Current signals with time of 2%MgCl2-SrCl2-KCl at different applied potentials
It can be seen from Fig. 3 that when cathode relative potential is -1.4 V or higher, the cathode has substantially no reduction current, so Mg2+ has not been precipitated yet. With the continuing negative shift of relative potential, when the cath ode relative potential reaches up to -1.6 V, a current signal appears and cathode starts to precip itate Mg2+. The amount of magnesium precipitation gradually increases with the continu ing negative shift of the cathode potential. When the cathode relative potential negative shifts over -2.2 V, cathode current increases signifi cantly. The main reason is that the cathode relative potential reaches the actual deposition potential of the Sr, and the underpotential precipitation of strontium on the liquid magne sium results in an obvious increase of the cath ode current.
The conditions of Mg and Sr codeposition can be judged from the chronoamperometry potential, namely when the cath ode relative potential reaches approxi mately -2.2 V, Mg and Sr will codeposit.
When the concentration of MgCl2 increases to 5%, chronoamperograms for 5%MgCl2-SrCl2-KCl melts at different applied potentials are shown in Fig. 4.
According to Fig. 4, when cathode relative po tential is -1.4 V or higher, the cathode has sub stantially no reduction current, so Mg2+ has not been precipitated yet. With the continuing nega tive shift of relative potential, when the cathode relative potential reaches up to -1.5 V, a current signal appears and cathode starts to precipitate Mg2+. The amount of magnesium precipitation gradually increases with the continuing negative shift of the cathode potential. When the cathode relative potential negative shifts over -2.1 V, cath ode current increases significantly. The main reason is that the cathode relative potential reaches the actual deposition potential of Sr, and the underpotential precipitation of Sr on the liquid Mg results in an obvious increase of the cathode current.
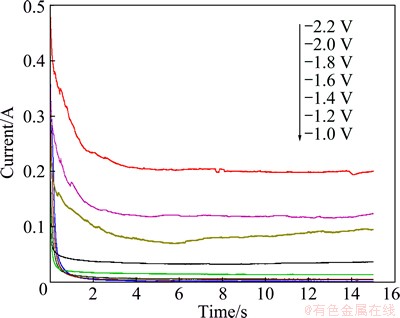
Fig. 4 Current signals with time of for 5%MgCl2-SrCl2-KCl at different applied potentials
The conditions of Mg and Sr codeposition can be judged from the chronoamperograms, namely when the cathode rela tive potential reaches approximately -2.1 V, Mg and Sr will codeposit.
Figure 5 shows the plot of I versus t-1/2 on a W electrode at different applied potentials in 5% MgCl2- SrCl2-KCl.
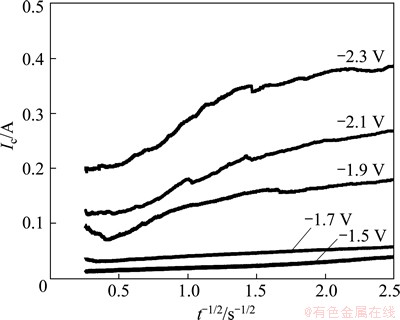
Fig. 5 Plot of I vs t-1/2 on W electrode at different applied potentials
From Fig. 5, I versus t-1/2 only establishes line arly proportional relationship in the range of -1.5 V to -1.7 V. There is only Mg2+ deposition, indicating that the Mg2+ reduction is a diffusion-controlled process [16]. When cathode rela tive potential reaches the deposition potential of Sr, I versus t-1/2 severely deviates from the linear relationship, which shows that it is no longer a diffusion-controlled process when Mg and Sr codeposit.
3.3 Chronopotentiometry
In order to study the electrochemical behav ior of Mg-Sr alloys by Mg-Sr codeposition further, chronopotentiograms obtained at different current intensities at 2% MgCl2-SrCl2-KCl melts are shown in Fig. 6.
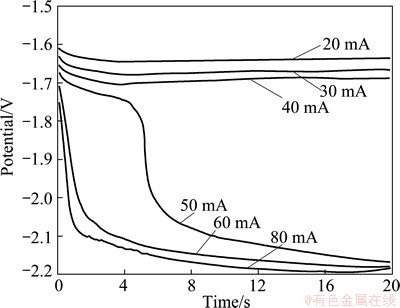
Fig. 6 Chronopotentiograms obtained at different currents in 2%MgCl2-SrCl2-KCl melts
According to Fig. 6, when the cathode cur rent intensity is more than 20 mA, the reduction potential platform of Mg2+ moves to the negative direction with the increase of current intensity. When the current exceeds 50 mA, the platform potential quickly negatively moves from -1.70 V to -2.10 V. This indicates that the cathode current intensity is more than 50 mA (the current density exceeds 0.71 A/cm2), the current density of Mg2+ is close to the limiting diffusion current density. Continuing to increase the current intensity, Sr2+ precipitates, and the potential platforms negatively moves to the strontium precipitate platform poten tial which is about -2.10 V. Meanwhile, since Mg and Sr can form an alloy phase with a different component, the activ ity of Sr greatly reduces, and its actual deposition potential also reduces.
The conditions of Mg and Sr codeposition can be judged from the chronopotentiograms, namely when the cathode current intensity is more than 50 mA (the current density exceeds 0.71 A/cm2), Mg and Sr will codeposit.
Increasing concentration of MgCl2 to 5%, chronopotentiograms obtained at different current intensities in 5% MgCl2-SrCl2-KCl melts are shown in Fig. 7.
According to Fig. 7, when the cathode cur rent intensity is more than 70 mA, the reduction potential platform of Mg2+ moves to the negative direction with the increase of current intensity. When the cathode current intensity is from 70 to 100 mA, the cathode relative potential reduces approximately -0.15 V. When the current exceeds 110 mA, the platform potential quickly negatively moves from -1.90 to -2.20 V. This indicates that the cathode current intensity is more than 110 mA (the current density exceeds 1.57 A/cm2), the current density of Mg2+ is close to the limiting diffusion current density. Continu ing to increase the current intensity, Sr2+ precipitates, and the potential platforms negatively moves to the Sr precipitate platform poten tial which is about -2.20 V. Meanwhile, as Mg and Sr can form an alloy phase with a different component, the activity of Sr greatly reduces and its actual deposition potential also reduces.

Fig. 7 Chronopotentiograms obtained at different current intensities in 5% MgCl2-SrCl2-KCl melts
The conditions of magnesium and stron tium codeposition can be judged from the chronopotentiograms, namely when the cathode current intensity is more than 110 mA (the cur rent density exceeds 1.57 A/cm2), Mg and Sr will codeposit.
Increasing concentration of MgCl2 to 10%, chronopotentiograms obtained at different current intensities in 10% MgCl2-SrCl2-KCl melts are shown in Fig. 8.
According to Fig. 8, when the cathode cur rent intensity is more than 140 mA, the reduction potential platform of Mg2+ moves to the negative direction with the increase of current intensity. When the cathode current intensity is in the range of 140-180 mA, the cathode relative potential reduces approximately -0.1 V. When the current exceeds 200 mA, the platform potential quickly negatively moved from -1.90 to -2.10 V. This indicates that the cathode current intensity is more than 200 mA (the current density exceeds 2.83 A/cm2), and the current density of Mg2+ is close to the limiting diffusion current density. Continu ing to increase the current intensity, Sr2+ precipitates, and the potential platforms negatively moves to the Sr precipitate platform poten tial which is about -2.10 V. Meanwhile, as Mg and Sr can form an alloy phase with a different component, the activity of Sr greatly reduces and its actual deposition potential also reduces.

Fig. 8 Chronopotentiograms obtained at different current intensities in 10% MgCl2-SrCl2-KCl melts
The conditions of Mg and Sr codeposition can be judged from the chronopotentiograms, namely when the cathode current intensity is more than 200 mA (the cur rent density exceeds 2.83 A/cm2), Mg and Sr will codeposit.
4 Conclusions
1) Liquid Mg-Sr alloys deposited at 700 °C as strontium deposited in magnesium (magnesium pre-deposited in W cathode) and formed underpotential deposition. The formation of Mg-Sr alloy reduced the activity of Sr and had depolarization effect, which made ac tual deposition potential of strontium reduce by about 0.5 V.
2) The electrochemical process of Mg-Sr was studied in MgCl2-SrCl2-KCl melts containing different MgCl2 concentrations at 700 °C by chronopotentiometry. The results showed that when cathode current densities are higher than 0.71, 1.57 and 2.83 A/cm2 in 2%, 5% and 10% MgCl2-SrCl2-KCl molten salts, respectively, Mg and Sr will codeposit.
3) It can be drawn from chronoamperometry that when the cathode relative potential is less than -1.5 V, Mg precipitates; When the cathode relative potential is below -2.0 V, Mg and Sr will co-precipitate. The chronoamperograms show that the entire elec trode process is no longer diffu sion-controlled in the co-precipitation of Mg and Sr.
References
[1] CUI Wei-hong, MIN Guang-hui, LIU Jun-cheng. Effect of trace element Sr on microstructure and properties of AM 60B magnesium alloy [J]. Rare Metal Materials and Engineer ing, 2010, 39(2): 273-276. (in Chinese)
[2] CHENG Ren-ju, PAN Fu-sheng, YANG Ming-bo, TANG Ai-tao. Effects of Mg-9Sr master alloys with different states on as-cast microstructure of AZ31 magnesium alloy [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(7): 1178-1184. (in Chinese)
[3] SHINA Sang-Soo, KIMA Eok-Soo, YEOM Gil-Yong, LEE Jae-Chul. Modifica tion effect of Sr on the microstructures and mechanical properties of Al-10.5Si-2.0Cu recycled alloy for die casting [J]. Materials Science and Engineering A, 2012, 532: 151-157.
[4] ARGYROPOULOS S A, CHOW G L S. An experimental investigation on the assimilation and recovery of strontium- magnesium alloy in A356 melts [J]. Journal of Light Metals, 2002, 2: 253-262.
[5] LIU Nian-chun, PENG Xiao-dong, XIE Wei-dong. Prepara tion of Mg-Sr alloy using electrochemical reduction [J]. Nonfer rous Metals, 2008(4): 44-50. (in Chinese)
[6] ZHANG Mi-lin, YAN Yong-de, HOU Zhi-yao, FAN Lu-an, CHEN Zeng, TANG Ding-xiang. Prepara tion of Mg-Li alloys by electrolysis in molten salt at low tempera ture [J]. Chinese Chemical Letters, 2007, 18: 329-332.
[7] YAN Yong-de, ZHANG Mi-lin, HAN Wei, XUE Yun, CAO Dian-xue, YUAN Yi. Electrochemical codeposition of Mg-Li alloys from a molten KCl-LiCl-MgCl2 system [J]. Chemistry Letters, 2008, 37(2): 212-213.
[8] WANG Yi-yong, LI Ji-dong, JIN Hui, WANG Zhi-ying. Preparation of Al-Ca master alloy by liquid aluminum cathode method [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(9): 2265-2269.
[9] ZHANG Mi-lin, CAO Peng, HAN Wei, YAN Yong-de, CHEN Li-jun. Preparation of Mg-Li-La alloys by electrolysis in molten salt [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 16-22.
[10] WEI Shu-quan, ZHANG Mi-lin, HAN Wei, YANG Yong-de, ZHANG Meng, ZHANG Bin. Electrochemi cal codeposition of Mg-Li-Gd alloys from LiC1-KC1-MgCl2-Gd2O3 melts [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(4): 825-829.
[11] LI Ya-ming, WANG Feng-li, ZHANG Mi-lin, HAN Wei, TIAN Yang. Study on electrochemical preparation of Al-Li-Y alloys from Y2O3 in LiCl-KCl-AlCl3 molten salts [J]. Journal of Rare Earths, 2011, 29(4): 378-382.
[12] CHEN Ye, YE Ke, ZHANG Mi-lin. Preparation of Mg-Yb alloy film by electrolysis in the molten LiCl-KCl-YbCl3 system at low temperature [J]. Journal of Rare Earths, 2010, 28(1): 128-133. (in Chinese)
[13] YANG Shao-hua, YANG Feng-li, LIAO Chun-fa, LI Ming-zhou, WANG Xu. Electrodeposition of magnesium-yttrium alloys by molten salt electrolysis [J]. Journal of Rare Earths, 2010, 28(S1): 385-388.
[14] LIU Song-qin, LU Qing-tao, JIN Zong-de, JIN Zong-de, CAO Li-xin. The electro chemical study on the dissolution behavior of Strontium in chlo ride melt [J]. Rare Metals, 1992, 1: 15-17.
[15] LU Gui-min, QIU Zhu-xian. Measurement of thermody namic properties of liquid Al-Mg alloys [J]. Transactions of Nonfer rous Metals Society of China, 1998, 8(1): 109-113.
[16] CHEN Zeng, ZHANG Min-li, HAN Wei, LI Sheng-jun, WANG Jun, YAN Yong-de, HOU Zhi-yao. Electrochemi cal reduction of Zr(IV)in the LiCl-KCl molten salt [J]. Rare Metal Materials and Engineer ing, 2009, 38(3): 456-459. (in Chinese).
熔盐电解共沉积Mg-Sr合金的电化学机理
孙秀云,路贵民,范书迪
华东理工大学 承压系统安全科学教育部重点实验室,
国家盐湖资源综合利用工程技术研究中心,上海 200237
摘 要:采用循环伏安法、计时电位法和计时电流法,研究在700 °C时不同MgCl浓度下,MgCl2-SrCl2-KCl熔盐体系中Mg和Sr共沉积的电化学过程。结果表明:由于Sr在Mg上形成欠电位沉积而形成液态Mg-Sr合金,从而使得Sr的实际析出电位降低0.5 V左右。在阴极相对电位低于-1.5 V左右时,Mg会析出,当阴极相对电位低于-2.0 V时,Mg和Sr会共析出。在Mg和Sr共析出时,整个电极过程不再是简单的扩散控制。计时电位法研究表明,Mg和Sr产生共电沉积的条件是在MgCl2浓度分别为2%、5%和10%(质量分数)的熔盐中,阴极电流密度分别为超过0.71、1.57和2.83 A/cm2。
关键词:熔盐电解;Mg-Sr合金;电化学还原
(Edited by Chao WANG)
Corresponding author: Xiu-yun SUN; Tel: +86-15921973312; E-mail: sxyun3@126.com
DOI: 10.1016/S1003-6326(14)63234-9