Catalytic performance and kinetics of Au/γ-Al2O3 catalysts forlow-temperature combustion of light alcohols
来源期刊:中国有色金属学报(英文版)2010年第3期
论文作者:邓谦 李小梅 彭振山 龙云飞 相龙明 蔡铁军
文章页码:437 - 442
Key words:gold; supported catalyst; light alcohols; low-temperature catalytic combustion; kinetics
Abstract: Au/γ-Al2O3 catalysts were prepared by deposition-precipitation method for the catalytic combustion of low concentration alcohol streams (methanol, ethanol, iso-propanol and n-propanol). The catalysts were characterized by X-ray photoelectron spectroscopy (XPS), X-ray diffractometry (XRD) and energy dispersive X-ray micro analysis (EDS) techniques. The XPS results showed that there was only Au0 on the surface of catalysts. The XRD patterns showed that Au was presumably highly dispersed over γ-Al2O3. The temperatures for complete conversion of methanol, ethanol, iso-propanol and n-propanol with concentration of 2.0 g/m3 were 60, 155, 170 and 137 ℃, respectively, but they were completely mineralized into CO2 and H2O at 60, 220, 260 and 217 ℃ respectively over the optimized catalyst. The activity of the catalyst was stable in 130 h. The kinetics for the catalytic methanol elimination followed quasi-first order reaction expressed as r=0.652 8c0+0.084 2. The value of apparent activation energy is 54.7 kJ/mol in the range of reaction temperature.

DENG Qian(邓 谦)1, LI Xiao-mei(李小梅)1, 2, PENG Zhen-shan(彭振山)1,
LONG Yun-fei(龙云飞)1, XIANG Long-ming(相龙明)2, CAI Tie-jun(蔡铁军)1
1. School of Chemistry and Chemical Engineering,
Key Laboratory of Theoretical Chemistry and Molecular Simulation, Ministry of Education,
Hunan University of Science and Technology, Xiangtan 411201, China;
2. Valiant Fine Chemicals Co. Ltd., Yantai 264006, China
Received 23 June 2009; accepted 25 August 2009
Abstract: Au/γ-Al2O3 catalysts were prepared by deposition-precipitation method for the catalytic combustion of low concentration alcohol streams (methanol, ethanol, iso-propanol and n-propanol). The catalysts were characterized by X-ray photoelectron spectroscopy (XPS), X-ray diffractometry (XRD) and energy dispersive X-ray micro analysis (EDS) techniques. The XPS results showed that there was only Au0 on the surface of catalysts. The XRD patterns showed that Au was presumably highly dispersed over γ-Al2O3. The temperatures for complete conversion of methanol, ethanol, iso-propanol and n-propanol with concentration of 2.0 g/m3 were 60, 155, 170 and 137 ℃, respectively, but they were completely mineralized into CO2 and H2O at 60, 220, 260 and 217 ℃ respectively over the optimized catalyst. The activity of the catalyst was stable in 130 h. The kinetics for the catalytic methanol elimination followed quasi-first order reaction expressed as r=0.652 8c0+0.084 2. The value of apparent activation energy is 54.7 kJ/mol in the range of reaction temperature.
Key words: gold; supported catalyst; light alcohols; low-temperature catalytic combustion; kinetics
1 Introduction
Light alcohols (such as methanol, ethanol, iso-propanol and n-propanol) belong to an important category of volatile, flammable chemical materials and solvents. Except for ethanol, the other three alcohols are very harmful to the human. In Chinese environment quantity standards (TJ36—79)[1], the maximum allowable concentrations of methanol in the air of residential and workshop areas are 0.003 g/m3 and 0.05 g/m3, respectively, and that for n-propanol is 0.2 g/m3 in the air of workshop. Thus, the elimination of these light alcohols in air is highly desirable. Generally, concentration of volatile organic compounds (VOCs) in industrial emissions is 0.1-2.0 g/m3. The low-temperature catalytic combustion method for the elimination of VOCs (with concentration less than 1%) has the advantages such as high efficiency, no secondary pollution and energy conservation.
In 1989, HARUTA et al[2] reported that 1.0 % CO in air can be totally oxidized to CO2 at temperature as low as -70 ℃ over supported nano gold catalyst on transition metal oxides. This greatly arises people’s interests in the catalysis property of gold. A lot of works on gold catalysts at low-temperature catalysis have been exhibited[3-6].
Supported gold catalysts prepared by deposition-precipitation and co-precipitation methods have high dispersion of gold, and the average particle size is less than 10.0 nm, surpassing the traditional impregnation method[7]. The gold catalysts on different carriers have different activities for elimination of the light alcohols at low temperature[8-9]. For example, over Au/Fe2O3 catalyst, the oxidation reaction of methanol starts at 80 ℃ and reaches total conversion at 160 ℃. But, on Au/CeO2 catalyst, the temperatures are 120 ℃ and 400 ℃, respectively. γ-Al2O3 has Lewis acid, Br?nsted acid and alkali centers on the surface simultaneously. High surface area makes it have strong adsorption performance. Thus, in this work, the γ-Al2O3 was chosen as the catalyst carrier to prepare Au/γ-Al2O3 catalysts by deposition-precipitation method for the low-temperature catalytic combustion of some representative VOCs (methanol ethanol, iso-propanol and n-propanol). The influence of preparation conditions and reaction conditions on the performance of Au/γ-Al2O3 catalysts was investigated. The kinetic behavior of methanol catalytic combustion was studied and the kinetics parameter was also estimated.
2 Experimental2.1 Catalyst preparation
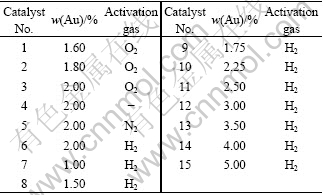
Supported Au/γ-Al2O3 catalysts were prepared via deposition-precipitation method[10]. A certain quantity of γ-Al2O3 was dispersed in distilled water in a beaker and heated to 70 ℃ under vigorous stirring. Thereafter, a certain volume of HAuCl4 aqueous solution was added drop-wise to the suspension. KOH aqueous solution (1 mol/L) was added drop-wise until pH value reached 7.0. The suspension was stirred and kept at 70 ℃ for 1 h. The sample was filtered and washed with distilled water until the filtrate has no Cl- detected by a AgNO3 solution. Finally, the sample was dried in air at 60 ℃ for at least 12 h and activated in a flow of pure H2, N2 and O2 at 300 ℃ for 1 h, respectively, to obtain the Au/γ-Al2O3 catalysts. Here, the Au loading was recorded as the gold amount added in the preparation process of catalysts. The gold loading and activation gas of Au/γ-Al2O3 catalysts are listed in Table 1.
Table 1 Designed Au loading and activation gas of Au/γ-Al2O3 catalysts
2.2 Catalyst characterization
X-ray diffractometry (XRD) was performed by a D/MAX 2500 powder X-ray diffractometer using Cu Kα radiation (λ=0.154 nm). The 2θ angles were scanned from 5? to 80? at a rate of 8 (?)/min. X-ray photoelectron spectroscopy (XPS) measurement was carried out on a Perkin-Elmer PHI Quantera XPS Scanning Microprobe with Al Kα radiation at room temperature. The shift of binding energy due to relative surface charging was corrected using the C 1s level at 284.8 eV as an internal standard.
Energy dispersive X-ray micro analysis (EDS) was conducted with GENESIS 60S energy dispersive X-ray spectroscopy instrument.
2.3 Catalytic reaction
The catalytic performance was measured in a continuous-flow fixed-bed micro-reactor filled with 1.0 g catalyst. The simulacrum of polluted air was prepared by bubbling clean air into a container filled with alcohol solution, and then diluting the gas with clean dry air. The initial alcohol concentration in the reaction gas was controlled by changing the proportion of the bubbling gas and diluent gas. The reactant mixture was fed to the tube reactor at a flow rate of 20.0 mL/min. The reaction temperature was raised up from 50 ℃ to a certain temperature at which alcohols were not detected in the effluent gases. The concentration of organic compounds of effluent gases was analyzed on-line by a gas chromatograph GC-9800, equipped with flame ionization detector (FID) through a packed column with Porapak-Q . The inorganic products CO and CO2 were monitored by 0.2% (mass fraction) PdCl2 solution and saturated limewater solution, respectively. The high-concentration alcohols were used to select the catalyst and to optimize the reaction condition; but the low-concentration alcohols were used to evaluate the catalytic performance of catalysts. Blank experiments were carried out without catalyst.
3 Results and discussion3.1 Characterization of catalysts
The XRD patterns (Fig.1) of the three samples showed that only peaks corresponding to AlO(OH) and Al2O3 phases existed; but those to the crystalline bulk gold (2θ=38.2?, 44.4?, 64.6?, 77.5?)[11-12] were not observed. This indicated that Au particle size is below the detection limit or Au has been doped into γ-Al2O3 lattice. So, Au is presumably highly dispersed over γ-Al2O3. The appropriate preparation methods (such as coprecipitation and deposition-precipitation method) can disperse gold on a number of metal oxides as reported in Ref.[13]. The Catalyst 6 for the elimination of the light alcohols has high activity, probably due to the gold cluster particles with size less than 10.0 nm.

Fig.1 XRD patterns of catalysts: 1—γ-Al2O3; 2—Catalyst 6 (fresh); 3—Catalyst 6 (after reaction)
The XRD diffraction peaks of the Catalyst 6 after methanol oxidation reaction for 10 h reveal no obvious change compared with fresh one, indicating that gold particles in the catalysts appear no aggregation and Catalyst 6 has relatively good stability to a certain extent.
XPS Au 4f7/2 spectra (Fig.2) of catalysts 4, 6, 3, 3 (after reaction) were located at 83.5, 83.4, 83.1, 82.7 eV, respectively, centered at 83.2±0.5, indicating the presence of Au0. But Au 4f7/2 peaks position shifted to lower energy with respect to that of pure gold (4f7/2 83.9 eV)[14].
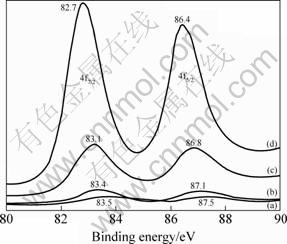
Fig.2 XPS spectra of Au/γ-Al2O3 catalysts: (a) Catalyst 4; (b) Catalyst 6; (c) Catalyst 3; (d) Catalyst 3 (after reaction)
When the electro-negativity of a metal is high, the reaction is characterized by a transfer of electrons from the oxygen to a metal. In this case, as the electro-negativity of Au (2.2 in the Pauling scale) is very high, a transfer of electrons from oxide (Al2O3) to the metal is possible. As the transfer of electrons from the matrix to gold particles is equivalent to the reduction process of gold, the lower energy shift of the Au 4f band is expected[15]. Due to the same reason, the formation of gold oxide is less possible. For the Catalyst 4, this result indicated that AuCl4- was gradually hydrolyzed into Au(OH)3 and deposited in the preparation solution. Au(OH)3 first dehydrates to unstable Au2O3 (ΔHf=19.3 kJ/mol), then decomposes into Au0 in the drying process[11]. For the catalysts 6 and 3, it is difficult to form a gold oxide, even at 300 ℃ in O2 atmosphere.
Another important influencing factor of the catalytic activity is the existence of the chlorine element[16]. EDS analysis confirmed that there was no chlorine element in catalyst.
3.2 Catalytic performance of gold catalysts
Blank experiments showed that the alcohols in simulacrum of polluted air were hardly eliminated without catalyst at 60 ℃.
The experimental results showed that catalytic activity of Au/γ-Al2O3 catalysts was all higher than that of γ-Al2O3, meaning that gold was the catalytic active center. The effluent gases made the saturated limewater muddy but did not make the color of the solution of 0.2% PdCl2 change, suggesting the formation of CO2 instead of CO. Different activation atmospheres had a little impact on the catalytic activity of the catalyst. Over the catalysts 3, 5 and 6 (gold content 2.0%, activated under O2, N2 and H2 atmosphere, respectively), the temperatures for the complete conversion of methanol with concentration of 2.0 g/m3 were 65, 63, 60 ℃, respectively. Thus, catalysts activated using H2 atmosphere were further investigated in the following experiments.
3.2.1 Catalytic activity for methanol elimination
The influence of Au loading on catalytic activity of the catalysts was tested and the results are summarized in Table 2.
Table 2 Catalytic performance of catalysts for methanol elimination
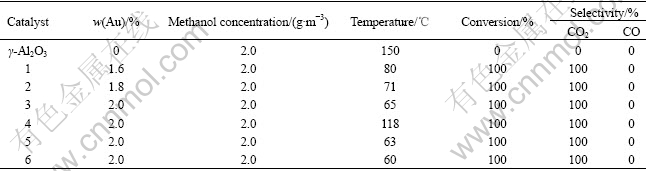
The selectivity of CO2 was almost 100% in methanol oxidation products over the catalysts 1-6. The temperature for complete elimination of methanol was lowered gradually with the increase of Au loading in the tested range. The lowest temperature (60 ℃) was achieved when the Au loading is 2.0%. The complete conversion temperature of the 86.3 g/m3 methanol is at 150 ℃ over Catalyst 6[10]. It is shown that Au0 seems to be the active species in accordance with XPS analysis. With the increase of Au loading, active sites on the catalyst surface increase, thus enhancing catalytic combustion activity.
Conversion of methanol as a function of concentration over catalyst 6 at 60 ℃ is shown in Fig.3. Methanol could be totally transformed into CO2 and H2O with a methanol concentration lower than 18.75 g/m3 at 60 ℃. When the methanol concentration was 30.0 g/m3, the conversion of methanol was only 93.3%, without other products besides CO2 and H2O at 60 ℃. Complete conversion was obtained at 70 ℃. It was shown that the largest methanol concentration of complete mineralization is 18.75 g/m3 over Catalyst 6 at 60 ℃. When methanol concentration was higher than 18.75 g/m3, the complete mineralization temperature should be increased.
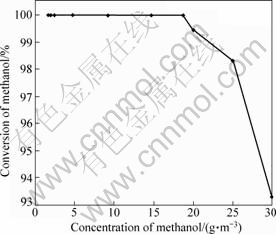
Fig.3 Conversion of methanol as function of its concentration on Catalyst 6
CORDI and FALCONER[17] observed that VOCs were adsorbed completely on Al2O3 over the Pd/Al2O3 and PdO/Al2O3 catalysts. The surface of Au may be inert for the adsorption of methanol. But O2 can be chemically adsorbed on gold clusters even at 0 ℃. The chemisorbed oxygen has Br?nsted alkalinity and can react with methanol[18]. Therefore, it is probable that O2 is first activated on the gold cluster and activated oxygen spills to the Al2O3 surface, then reacts with the adsorptive methanol to form CO2 and H2O. The activated oxygen species are not able to form on the Al2O3 surface.
To test the stability of the catalyst, Catalyst 6 as the optimized catalyst was tested. Conversion of methanol maintained about 100% for 130 h with a concentration of 2.0 g/m3 at 60 ℃, showing a better stability.
3.2.2 Catalytic activity for ethanol elimination
Catalytic elimination of ethanol with concentrations of 52.2 g/m3 and 2.0 g/m3 was carried out. The influence of Au loading on activity for ethanol elimination was tested and the results are summarized in Table 3.
Table 3 Catalytic performance of catalysts to ethanol elimination
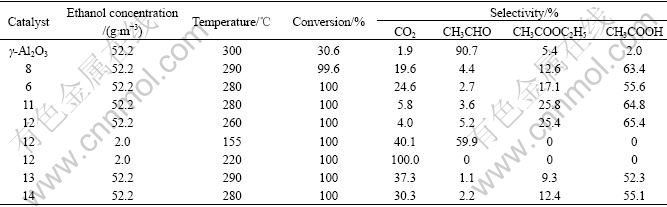
The Catalyst 12 (gold content 3.0%) had the best activity, with a complete elimination temperature of ethanol at 260 ℃ for concentration of 52.2 g/m3. Different from methanol, the oxidation reaction of ethanol produced acetaldehyde, ethyl acetate and acetic acid. The total selectivity of three organic products was higher than 60%. On γ-Al2O3, selectivity of acetaldehyde was as high as 90.7%, and only a small quantity of ethyl acetate and acetic acid was formed. On the contrary, the selectivity of acetic acid was the highest over Au/γ-Al2O3. Consequently, the selectivity of acetic acid over Catalyst 12 reached 65.4%, showing that presence of gold enhanced deep oxidation of ethanol. When ethanol concentration was 2.0 g/m3, complete conversion temperature of ethanol was 155 ℃, with acetaldehyde as the only organic product over the optimal catalyst (Catalyst 12); and the mineralization temperature of ethanol was 220 ℃.
3.2.3 Catalytic activity for iso-propanol elimination
Catalytic elimination of iso-propanol with concentrations of 57.7 g/m3 and 2.0 g/m3 was tested. The influence of Au loading on the catalytic activity of the catalysts is summarized in Table 4.
Table 4 Catalytic performance of catalysts for iso-propanol elimination
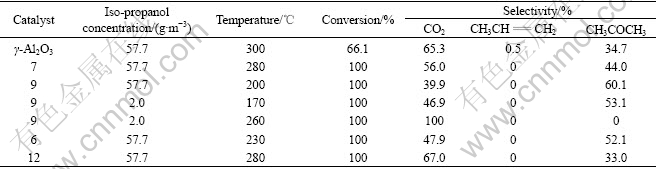
The Catalyst 9 had the best activity, with complete conversion temperature of 57.7 g/m3 iso-propanol at 200 ℃. Acetone was the only by-product. Whereas, propene was produced on γ-Al2O3. The selectivity of acetone was the highest on Catalyst 9, reaching 60.1%. When iso-propanol concentration was 2.0 g/m3, the complete conversion temperature of iso-propanol was 170 ℃, with acetone as the only organic product over the optimized catalyst (Catalyst 9); and the mineralization temperature of iso-propanol was 260 ℃.
3.2.4 Catalytic activity for n-propanol elimination
Catalytic combustion of n-propanol with concentrations of 79.8 g/m3 and 2.0 g/m3 was tested. The influence of Au loading on the catalytic activity is summarized in Table 5.
Table 5 Catalytic performance for n-propanol elimination

The catalysts 13 and 14 had good activity with complete conversion temperature of 79.8 g/m3 n-propanol at 260 ℃. The formations of propene and propanal were also observed on all catalysts. The selectivity of CO2 was the highest (84.5%) over Catalyst 12, while propanal was the lowest (14.2%). When n-propanol concentration was 2.0 g/m3, the complete conversion temperature of n-propanol was 137℃, with propylene and propanal as the organic products over the optimal catalyst (catalyst 13), obtaining 100% oxidation into CO2 and H2O at 217 ℃.
To sum up, for different alcohols, the best catalyst was different in Au loading. For the elimination of methanol, ethanol, iso-propanol and n-propanol, the best catalytic activity was for the catalysts in which Au loadings were 2.0%, 3.0%, 1.75% and 3.5%, respectively.
3.3 Reaction kinetics of methanol catalytic elimination
If the flow method was used to evaluate the activity of the catalyst, the influence of internal and external diffusion must be excluded in order to gain correct kinetics model. In order to exclude diffusion effects, the test conditions of the methanol elimination over Au/γ-Al2O3 were investigated. According to Ref.[19], catalytic reactions were carried out at different volume flow velocities (0.17-0.5 mL/s) and catalyst granular sizes (0.1-0.2, 0.2-0.3, 0.3-0.4, 0.4-0.5, 1.0-1.6 mm). The results showed that when the volume flow velocity was in the range of 0.17-0.5 mL/s and catalyst granular size was less than 0.5 mm, diffusion limitation was excluded. Thus, experimental parameters were controlled strictly in accordance with the requirements.
By changing the initial concentration of methanol, the reaction rate at the volume flow rate of 0.33 mL/s over 0.48 mL catalyst 6 was measured. The relationship between methanol catalytic elimination reaction velocity (r) and methanol initial concentration (c0) is shown in Fig.4.

Fig.4 Reaction rate (r) as function of methanol initial concentration
When the methanol initial concentration was in the range of (0. 625-9.375)×10-4 mol/L (2.0-30.0 g/m3), r had a linear relationship with c0, as expressed by the equation r=0.652 8c0+0.084 2, with fitting relevant factor R=0.9987. O2 concentration in the feed gas was basically invariable in the process of elimination. Therefore, the reaction rate was in quasi-first order, r=kc, where c is the concentration. When substituting equation r=kc into the basic calculation formula of integral reactor, and then integrating, the following equation was obtained[20]:
![]() (1)
(1)
where V is the volume of catalyst, F is molar velocity and x is conversion of methanol. When methanol initial concentration was 7.813×10-4 mol/L (25 g/m3), the molar flow velocity was 2.60×10-7mol/s (the volume flow velocity is 0.33 mL/s). The conversion of methanol was measured at different reaction temperatures over the 0.48 mL Catalyst 6. When reaction temperatures were 55, 60 and 63 ℃, the methanol conversion rates were 95.49%, 98.53% and 99.34%, respectively. Substituting these data into Eq.(1), k1=2.15 s-1, k2=2.85 s-1, k3=3.48 s-1 were obtained.
Using lnk and 1/T to plot according to the Arrhenius equation: ![]() , lnk=-6 582.8/T+20.83 was
, lnk=-6 582.8/T+20.83 was
obtained with fitting correlation coefficient R=0.998 6. The apparent activity energy (Ea) was estimated to be 54.7 kJ/mol. In the tested temperature range, the apparent activation energy Ea had nothing to do with the temperature.
4 Conclusions1) Au/γ-Al2O3 catalysts prepared by deposition- precipitation method exerted a good catalytic activity for the catalytic combustion of light alcohol stream. The temperatures for complete conversion of methanol, ethanol, iso-propanol and n-propanol with concentration of 2.0 g/m3 were 60, 155, 170 and 137 ℃, respectively, over the optimized catalyst.
2) The kinetics for the catalytic methanol elimination followed quasi-first order reaction expressed as r=0.652 8c0+0.084 2. The value of apparent activation energy was 54.7 kJ/mol in the range of reaction temperature.
References[1] States Environmental Protection Agency Office of the Management of Toxic Chemicals, Beijing Research Institute of Chemical Industry Environmental Protection Institute. Toxic chemical’s, toxicity, law and environmental data manual [M]. Beijing: China Environmental Science Press, 1992. (in Chinese)
[2] HARUTA M, YAMADA N, KOBAYASHI T, LIJIMA S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide [J]. Journal of Catalysis, 1989, 115: 301-309.
[3] HUANG X S, SUN H, WANG L C, LIU Y M, FAN K N, CAO Y. Morphology effects of nanoscale ceria on the activity of Au/CeO2 catalysts for low-temperature CO oxidation [J].Applied Catalysis B: Environmental, 2009, 90: 224-232.
[4] MOROZ B L, PYRJAEV P A, ZAIKOVSKII V I, UKHTIYAROV V I. Nanodispersed Au/Al2O3 catalysts for low-temperature CO oxidation: Results of research activity at the Boreskov Institute of Catalysis [J]. Catalysis Today, 2009, 144: 292-305.
[5] WEN L, FU J K, GU P Y, YAO B X, LIN Z H, ZHOU J Z. Monodispersed gold nanoparticles supported on g-Al2O3 for enhancement of low-temperature catalytic oxidation of CO [J]. Applied Catalysis B: Environmental, 2008, 79: 402-409.
[6] VEITH G M, LUPINI A R, RASHKEEV S, PENNYCOOK S J, MULLINS D R, SCHWARTZ V, BRIDGES C A, DUDNEY N J. Thermal stability and catalytic activity of gold nanoparticles supported on silica [J]. Journal of Catalysis, 2009, 262: 92-101.
[7] GRISEL R J H, KOOYMAN P J, NIEUWENHUYS B E. Influence of the preparation of Au/Al2O3 on CH4 oxidation activity [J]. Journal of Catalysis, 2000, 191: 430-437.
[8] MINIC? S, SCOR? S, CRISAFULLI C, MAGGIORE R, GALVAGNO S. Catalytic combustion of volatile organic compounds on gold/iron oxide catalysts[J]. Applied Catalysis B, 2000, 28: 245-251.
[9] SCIR? S, MINIC? S, CRISAFULLI C, SATRIANO C, PISTONE A. Catalytic combustion of volatile organic compounds on gold/cerium oxide catalysts [J]. Applied Catalysis B, 2003, 40: 43-49.
[10] DENG Qian, LI Xiao-mei, CAI Tie-jun, PENG Zhen-shan, YUE Ming. The study of catalytic elimination of high concentration methanol on supported Au/γ-Al2O3 catalysts [J]. J Hunan Univ Sci? Technol, 2006, 21(3): 77-80. (in Chinese)
[11] CENTENO M A, PAULIS M, MONTES M, ODRIOZOLA J A. Catalytic combustion of volatile organic compounds on Au/CeO2/Al2O3 and Au/Al2O3 catalysts [J]. Applied Catalysis A, 2002, 234: 65-78.
[12] LEE S J, GAVRIILIDIS A. Supported Au catalysts for low-temperature CO oxidation prepared by impregnation [J]. Journal of Catalysis, 2002, 206: 305-313.
[13] SCOR? S, MINIC? S, CRISAFULLI C, GALVAGNO S. Influence of catalyst pretreatments on volatile organic compounds oxidation over gold/iron oxide [J]. Applied Catalysis B, 2001, 34: 277-285.
[14] PARK E D, LEE J S. Effects of pretreatment conditions on CO oxidation over supported Au catalysts [J]. Journal of Catalysis, 1999, 186: 1-11.
[15] SERRANO J G, GALINDO A G, PAL U. Au-Al2O3 nanocomposites: XPS and FTIR pectroscopic studies [J]. Solar Energy Materials & Solar Cells, 2004, 82: 291-298.
[16] OH H S, YANG J H, COSTELLO C K, BARE S R, KUNG H H, KUNG M C. Selective catalytic oxidation of CO: Effect of chloride on supported Au catalysts [J]. Journal of Catalysis, 2002, 210: 375-386.
[17] CORDI E M, FALCONER J L. Oxidation of volatile organic compounds on Al2O3, Pd/Al2O3, and PdO/Al2O3 catalysts [J]. Journal of Catalysis, 1996, 162: 104-107.
[18] HARUTA M. Size-and support-dependency in the catalysis of gold [J]. Catalysis Today, 1997, 36: 153-166
[19] WANG Shang-di, SHUN Jun-quan. Catalyst engineering introduction [M]. Beijing: Chemistry Industry Press, 2001: 113-115. (in Chinese)
[20] LIU Dan-chu. Principle of heterogeneous catalysis [M]. Shanghai: Fudan University Press, 1997: 20-21. (in Chinese)
Foundation item: Project supported by the Key Laboratory of Theoretical Chemistry and Molecular Simulation of Ministry of Education, China
Corresponding author: DENG Qian; Tel: +86-13170324060; E-mail: dengqian10@126.com
DOI: 10.1016/S1003-6326(09)60159-X

