Trans. Nonferrous Met. Soc. China 25(2015) 314-318
Flotation of carbonaceous copper shale-quartz mixture with poly(ethylene glycol) alkyl ethers
Przemyslaw B. KOWALCZUK, Emilia ZALESKA, Oliver DANCZAK
Wroclaw University of Technology, Wybrzeze Wyspianskiego 27, Wroclaw 50-370, Poland
Received 21 February 2014; accepted 7 June 2014
Abstract: The influence of different poly(ethylene glycol) alkyl ethers (CnH2n+1O(C2H4O)mH, CnEm) on flotation of carbonaceous copper shale mixed with quartz as a gangue mineral was investigated. The results show that all of the ethers C4E1, C4E2, C4E3, C2E2, C6E2 investigated can be used for the flotation of carbonaceous copper shale. The best selectivity of separation in the flotation of the carbonaceous copper shale and quartz mixture is obtained with the C4E2 and C2E2 ethers. The obtained data can be used for developing separation of organic carbon present in carbonaceous shale at a rougher flotation stage on an industrial scale.
Key words: flotation; frother; selectivity; shale; quartz; poly(ethylene glycol) alkyl ethers
1 Introduction
Flotation is a physico-chemical process commonly used for upgrading of ores and other materials. This process is used to separate valuable materials from unwanted ones utilizing the differences of their surface properties. These properties can be modified using different chemical reagents. Since collectors are adsorbed at the solid/liquid interface, they are used to promote successful attachment of a valuable particle to a bubble and form a stable particle-bubble aggregate [1,2]. On the other hand, depressors are used to make the unwanted particles hydrophilic. While the collectors influence particle hydrophobicity, the role of frothers, which are adsorbed mainly at the liquid/gas interface, is to prevent the bubbles coalescence and stabilize the bubble attachment in the pulp froth layer, and therefore to enhance the efficiency of flotation process [3,4].
It is well established that the type, chemical structure and amount of reagent are very important in froth flotation providing different flotation recoveries of the solid particles [5-8]. The chemical reagents used as collectors can be characterized by hydrophobicity (contact angle) of solid particles, while frothers are mostly characterized by their relative molecular mass, hydrophilic-lipophilic balance (HLB), dynamic foamability index (DFI), critical coalescence concentration (CCC) and reagent dosage (C) [3,9,10]. Some flotation frothers can act both as frothers and collectors when they are able to be adsorbed not only at the liquid/gas interface but also at solid/gas and solid/water interfaces, rendering the solid particles hydrophobic and floatable [11]. One group of such reagents exhibiting adsorption onto selected solid surfaces are poly(ethylene glycol) alkyl ethers (CnH2n+1O(C2H4O)mH, CnEm), which are used for upgrading of different materials, including coal [12-14], graphite [15], quartz [16-18] and phosphate ores [19].
In this work, the influence of selected poly (ethylene glycol) alkyl ethers (CnH2n+1O(C2H4O)mH, CnEm) on the flotation performance of a model mixture of carbonaceous copper shale and quartz as a gangue material was investigated. The experiments were performed to establish a better understanding of the role of CnEm ethers in froth flotation.
2 Experimental
Flotation tests were carried out in a Mekhanobr laboratory flotation machine equipped with a 0.25 L cell. A geological sample of carbonaceous copper shale was originated from the Kupferschiefer stratiform copper ore (Legnica-Glogow Zechstein Copper Basin LGOM), while quartz (98% SiO2, 0.05% Fe2O3, 0.3% TiO2) was originated from the Osiecznica Mine, located in southwestern Poland. Advancing and receding contact angles for the air/shale/water system were 42° and 24°, respectively. A 70 g of mixture of carbonaceous copper shale (10 g) and quartz (60 g), both with the narrow size fraction of 40-100 μm, and distilled water were mixed together and agitated for 2 min in the flotation cell before adding any reagent. In each test, the air flow rate was 10 L/h and the stirring speed was 1200 r/min. The experiments were performed under natural pH conditions. The samples were floated in the presence of different poly(ethylene glycol) alkyl ethers (CnH2n+1O(C2H4O)mH, CnEm) at three concentrations equal to 0.028, 0.070 and 0.112 mmol/L. After the reagent addition, the pulp was conditioned for 5 min. The concentrates were collected after 1, 3 and 7 min as the froth products. The total time of flotation was 7 min in each test. The flotation products (froth products and tailing) were dried in an oven at 100 °C for 24 h and then weighed to determine the concentrate yield. Since carbonaceous copper shale (black) and quartz (white) varied in colour, their contents in the flotation products were determined using a Motic SFC-11 microscope.
The flotation reagents used in this work were obtained from Sigma-Aldrich (≥99% purity) and were used without further purification. Table 1 gives the properties of the poly(ethylene glycol) alkyl ethers (CnH2n+1O(C2H4O)mH, CnEm) family including the numbers of alkyl (n) and ethylene glycol (m) groups, hydrophilic-lipophilic balance (HLB), relative molecular mass (M) and critical coalescence concentration (CCC95).
Table 1 Properties of poly(ethylene glycol) ethers (CnH2n+1O(C2H4O)mH, CnEm)

The value of the hydrophilic-lipophilic (hydrophobic) balance depends on the number of hydrophilic and hydrophobic groups in the molecule and can be calculated using a formula proposed by DAVIES [20]: HLB=7+1.3n(O)+1.9n(OH)-0.475n(CxHy), where n(O) and n(OH) are the numbers of hydrophilic oxygen and hydroxyl functional groups, and n(CxHy) stands for the numbers of hydrophobic (lipophilic) —CH, —CH2—, —CH3—, and =CH— groups. Table 1 gives that higher numbers of lipophilic groups cause higher values of HLB. For all considered ethers in this work, the HLB values exceed 5.1, indicating that they all are frothers.
The critical coalescence concentration, expressed as CCC95, characterizes the reagent ability to prevent the bubble coalescence [3]. The values of CCC95 are estimated based on the chemical structure of the frother, that is its relative molecular mass (M) and hydrophilic- lipophilic balance [10]. The CCC95 indicates the frother concentration at which there is a 95% reduction in the mean bubble size in comparison to the mean bubble size in water only.
3 Results and discussion
The flotation tests were performed to investigate the influence of the type and dosage of selected poly (ethylene glycol) alkyl ethers on the flotation performance of a model mixture of carbonaceous copper shale and quartz. The results in the form of concentrate yield versus ether concentration are given in Fig. 1. It can be seen that three ethers, that is di(ethylene glycol) monohexyl C6E2, tri(ethylene glycol) monobutyl C4E3 and mono(ethylene glycol) monobutyl C4E1 follow a similar pattern and form one family of lines. Figure 1 shows that the concentrate yield increases with concentration expressed both in mmol/L and μg/g. The maximum yield value of 20% is obtained for C6E2. For di(ethylene glycol) monoethyl C2E2 and di(ethylene glycol) monobutyl C4E2 ethers, the concentrate yield remains almost constant and is equal to 10%. The results indicate that all the ethers investigated in this work improve the flotation up to a certain plateau level.
Figure 2(a) shows the influence of poly(ethylene glycol) alkyl ethers on the recovery of carbonaceous copper shale. It can be seen that all ethers form one family of lines and exhibit very high collecting properties of carbonaceous copper shale and the best results, exceeding 90% in recovery, are obtained for mono(ethylene glycol) monobutyl ether C4E1.
The influence of reagent concentration on the recovery of quartz, which is the gangue material, is shown in Fig. 2(a). Figure 2 indicates that three ethers, that is di(ethylene glycol) monohexyl C6E2, tri(ethylene glycol) monobutyl C4E3 and mono(ethylene glycol) monobutyl C4E1 ethers improve flotation of quartz and its recovery increases with concentration. For the other two ethers, that is di(ethylene glycol) monoethyl C2E2 and di(ethylene glycol) monobutyl C4E2, the recovery of quartz is very low and remains constant with the reagent concentration. It means that among all ethers tested in this work, the latters can be used as selective ones during the flotation of materials consisting of quartz and carbonaceous copper shale.
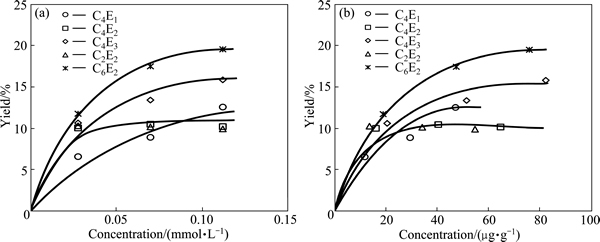
Fig. 1 Influence of ether concentration, expressed as mmol/L (a) and μg/g (b), on concentrate yield

Fig. 2 Influence of ether concentration on recovery of shale (a) and quartz (b)
To check the selectivity of flotation in the presence of the ethers, the results are plotted as a relationship between the recovery of shale in the concentrate and the recovery of quartz in the tailing. Such a relationship is also known as the Fuerstenau upgrading curve [21]. The upgrading curve for C4E3, as an example, is given in Fig. 3, where the solid points show the experimental data for the used concentration of 0.028, 0.070 and 0.112 mmol/L, respectively. The best approximation of the experimental points is obtained by using a nonlinear last-squares regression for one-adjustable parameter, called selectivity A, and has the form [22]:
 (1)
(1)
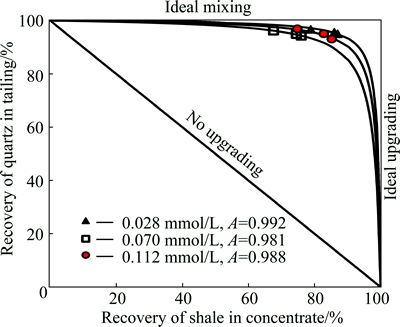
Fig. 3 Recovery of quartz in tailing versus recovery of carbonaceous copper shale in concentrate
where εq,t is the recovery of quartz in the tailing and εs,c is the recovery of shale in the concentrate. Selectivity A assumes values from 0 for non-selective flotation to 1 for an ideal upgrading process. Equation (1) and the Fuerstenau plot can be used to check the selectivity of any reagent used in flotation.
The selectivity of ethers in flotation of the mixture of carbonaceous copper shale and quartz is shown in Fig. 4 as a function of concentration expressed in mmol/L (Fig. 4(a)) and μg/g (Fig. 4(b)). It can be seen that the selectivity decreases with increasing frother dosage for C4E1, C4E3 and C6E2 and increases for C2E2 and remains constant or slightly increases for C4E2. It confirms that the used ethers form two groups of reagents. One of them is a group of the C4E1, C4E3 and C6E2 ethers, which provide the best selectivity of separation between shale and quartz, especially at low concentrations. Incidentally, C4E3 and C6E2 have relatively low values of the critical coalescence concentration CCC95 (Table 1). The second group is formed by the C4E2 and C2E2 ethers, for which the highest values of selectivity index A are obtained for relatively high values of ether concentration.
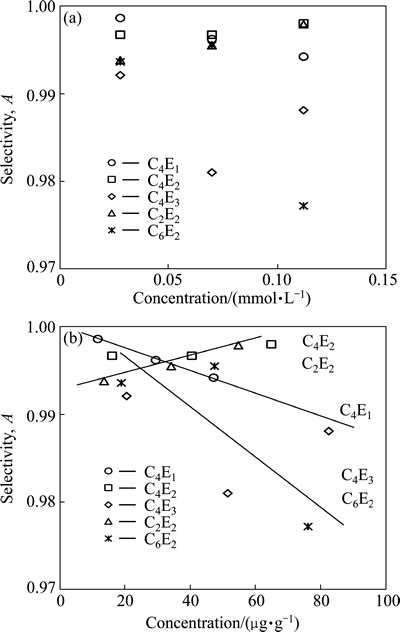
Fig. 4 Influence of ether concentration on selectivity of carbonaceous copper shale and quartz mixture expressed in mmol/L (a) and μg/g (b)
The influence of quartz recovery on the selectivity of flotation of the carbonaceous copper shale and quartz mixture is given in Fig. 5. It can be seen in Fig. 5 that the selectivity decreases with quartz recovery in the concentrate for all frothers at the used concentrations of frothers equal to 0.028, 0.070 and 0.112 mmol/L.

Fig. 5 Selectivity versus quartz recovery in concentrate
4 Conclusions
The influence of the type and dosage of poly(ethylene glycol) alkyl ethers on the flotation performance of model mixtures of carbonaceous copper shale and quartz was investigated. The results clearly show that all the used ethers, that is mono(ethylene glycol) monobutyl C4E1, di(ethylene glycol) monoethyl C2E2, di(ethylene glycol) monobutyl C4E2, di(ethylene glycol) monohexyl C6E2, tri(ethylene glycol) monobutyl C4E3 can be used for separation of carbonaceous copper shale from quartz by flotation. It is also established that the highest recoveries of quartz are obtained for C4E1, C4E3 and C6E2 ethers. The best selectivity of flotation of the carbonaceous copper shale and quartz mixture is observed for C4E2 and C2E2 ethers, which do not enhance quartz flotation. The obtained results can be used, for instance, to develop a technology for separation of organic carbon present in carbonaceous shale.
Acknowledgements
The authors thank Prof. Andrzej LUSZCZKIEWICZ (Wroclaw University of Technology) for his help in many aspects of this work. Financial support by the National Science Centre Research Grant (2012/07/D/ST8/02622) and a fellowships financed by the Foundation for Polish Science (FNP) and the European Union within the European Social Fund are also greatly acknowledged.
References
[1] DRZYMALA J. Mineral processing: Foundations of theory and practice of minerallurgy [M]. Wroclaw: Oficyna Wydawnicza PWr, 2007.
[2] WIESE J, HARRIS P, BRADSHAW D. The effect of the reagent suite on froth stability in laboratory scale batch flotation tests [J]. Minerals Engineering, 2011, 24: 995-1003.
[3] CHO Y S, LASKOWSKI J S. Effect of flotation frothers on bubble size and foam stability [J]. International Journal of Mineral Processing, 2002, 64: 69-80.
[4] BHATTACHARYA S, DEY S. Evaluation of frother performance in coal flotation: A critical review of existing methodologies [J]. Mineral Processing and Extractive Metallurgy Review, 2008, 29(4): 275-298.
[5] SALEH AM, ISKRA J. Effect of molecular weight of polyethylene glycol frothers in their performance in low rank coal flotation [J]. Physicochemical Problems of Mineral Processing, 1996, 30: 33-40.
[6] LASKOWSKI J S. Coal flotation and fine coal utilization [M]//Developments in Mineral Processing. Vol. 14, Amsterdam, The Netherlands: Elsevier Science Publishing, 2001.
[7] FARROKHPAY S. The significance of froth stability in mineral flotation—A review [J]. Advances in Colloid and Interface Science, 2011, 166: 1-7.
[8] GU Y, FENG Q, OU L,  P. A new method of testing frother performance [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 2776-2780.
P. A new method of testing frother performance [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 2776-2780.
[9] CZARNECKI J, MALYSA K, POMIANOWSKI A. Dynamic frothability index [J]. Journal of Colloid and Interface Science, 1982, 86: 570-572.
[10] KOWALCZUK P B. Determination of critical coalescence concentration and bubble size for surfactants used as flotation frothers [J]. Industrial & Engineering Chemistry Research, 2013, 52(33): 11752-11757.
[11] HARRIS G H, JIA R. An improved class of flotation frothers [J]. International Journal of Mineral Processing, 2000, 58(1-4): 35-43.
[12] JIA R, HARRIS G H, FUERSTENAU D W. An improved class of universal collectors for the flotation of oxidized and/or low rank coal [J]. International Journal of Mineral Processing, 2000, 58(1-4): 99-118.
[13] OZMAK M, AKTAS Z. Coal froth flotation: Effect of reagent adsorption on the froth stricture [J]. Energy & Fuels, 2006, 20: 1123-1130.
[14] GUPTA A K, BANERJEE P K, MISHRA A, SATISH P, PRADIP. Effect of alcohol and polyglycol ether frothers on foam stability, bubble size and coal flotation [J]. International Journal of Mineral Processing, 2007, 82: 126-137.
[15] PUGH RJ. Non-ionic polyethylene oxide frothers in graphite flotation [J]. Minerals Engineering, 2000, 13(2): 161-162.
[16] MATHUR S, SINGH P, MOUDGIL B M. Advances in selective flocculation technology for solid-solid separations [J]. International Journal of Mineral Processing, 2000, 58(1-4): 201-222.
[17] DRZYMALA J, MILCZARSKI E, MILCZARSKI J. Adsorption and flotation of hydrophilic and hydrophobic materials in the presence of hydrocarbon polyethylene glycol ethers [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 308(1-3): 111-117.
[18] GONG J, PENG Y, BOUAJILA A, OURRIBAN M, YEUNG A, LIU Q. Reducing quartz gangue entrainment in sulphite ore flotation by high molecular weight polyethylene oxide [J]. International Journal of Mineral Processing, 2010, 97: 44-51.
[19] SIS H, CHANDER S. Improving froth characteristics and flotation recovery of phosphate ores with nonionic surfactants [J]. Minerals Engineering, 2003, 16: 587-595.
[20] DAVIES J T. A quantitative kinetic theory of emulsion type, I. Physical chemistry of the emulsifying agent [C]//Proceedings of 2nd International Congress of Surface Activity. London, 1957: 426-438.
[21] DRZYMALA J, AHMED H A M. Mathematical equations for approximation of separation results using the Fuerstenau upgrading curves [J]. International Journal of Mineral Processing, 2005, 76: 55-65.
[22] AHMED H A M. Application of microemulsion for upgrading difficult-to-float materials [D]. Wroclaw: Wroclaw University of Technology, 2005.
碳质铜页岩-石英混合物的聚(乙二醇)烷基醚浮选
Przemyslaw B. KOWALCZUK, Emilia ZALESKA, Oliver DANCZAK
Wroclaw University of Technology, Wybrzeze Wyspianskiego 27, Wroclaw 50-370, Poland
摘 要:研究不同聚(乙二醇)烷基醚(CnH2n+1O(C2H4O)m, CnEm))对碳质铜页岩-石英混合物浮选的影响。研究结果表明:所研究的聚(乙二醇)烷基醚(C4E1,C4E2,C4E3,C2E2,C6E2)都可以作为碳质铜页岩的浮选剂。C4E2和C2E2在碳质铜页岩和石英混合物的分离浮选过程中具有最佳的选出率。研究结果可为工业初选阶段分离碳质铜页岩中的有机碳提供参考。
关键词:浮选;起泡剂;选出率;页岩;石英;聚(乙二醇)烷基醚
(Edited by Yun-bin HE)
Corresponding author: Przemyslaw B. KOWALCZUK; Tel: +48-713206864; E-mail: przemyslaw.kowalczuk@pwr.edu.pl
DOI: 10.1016/S1003-6326(15)63606-8