(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: The specific heat capacities at constant pressure of alkaline sodium aluminate solutions in NaOH-NaAl(OH)4- H2O system were determined using a commercial calorimeter (Setaram C80) at temperatures ranging from 298.15 K to 363.15 K and constant pressure, where the total alkli molality mT(mNaOH+mNaAl(OH)4) and αK(mT/mNaAl(OH)4) are 0.88-6.16 mol/kg and 1.9-5.0, respectively. The equation of the specific heat capacity as a function of total alkalinity, αK and temperature was constructed for sodium aluminate solution. The error between the results calculated from this equation and experimental values derived from the literature is 0.014. The apparent molar specific heat capacities (cpΦ) for sodium aluminate solution were also calculated, and the results show that the apparent molar specific heat capacities (cpΦ) have a maximum value with the temperature increasing, and that cpΦ is linear varied with the values of 1/αK.
Key words: sodium aluminate solution; ternary system; heat capacity; apparent molar heat capacities
实验的参比池和样品池都使用常压标准池。由于铝酸钠溶液具有强碱性,所以在常压池内加聚乙烯塑料管作为内衬。采用台阶升温法测定溶液的比定压热容:在样品池中加入6 g左右铝酸钠溶液,参比池不加溶液,量热仪在初始温度T0达到稳定后,以0.25 K/min的升温速率升温到T1,并恒温1 h,得到样品有个吸热量Q1,再以同样升温速率升温到T2,并恒温同样时间,得到样品的吸热量Q2,依次类推,至最终温度(363.15 K)。当样品池和参比池都为空时采用以上同样操作,则理论上热效应应为零,但由于仪器本身的原因,空白测空的热效应不为零,因此,需将此空白热效应从样品测量结果中扣除。
1) M(aq) is solution mass.
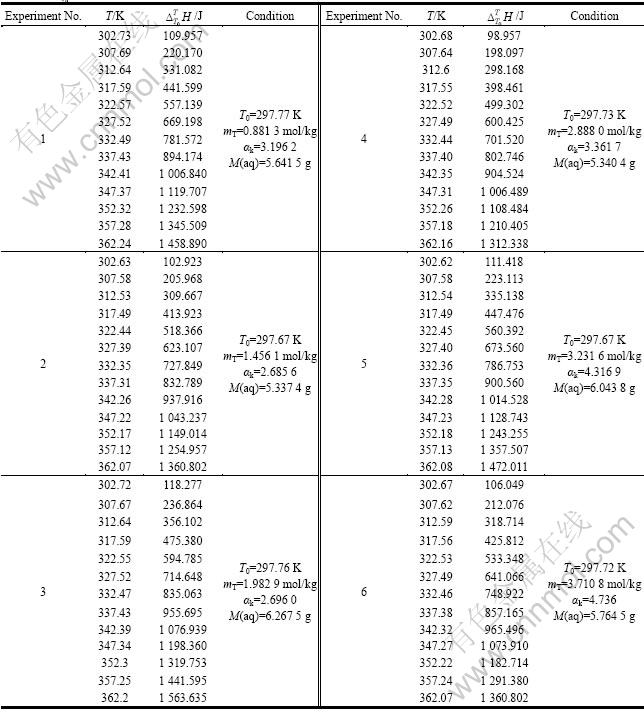
表3 不同浓度铝酸钠溶液的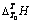
Table 3 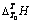 of sodium aluminate solution with different concentrations
of sodium aluminate solution with different concentrations

实验1~10号所采用的空白在温度为298.15~ 363.15 K常压条件下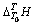 与T的关系式为
与T的关系式为
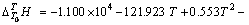
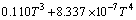 (3)
(3)
实验11、12号所采用的空白在温度为298.15~ 363.15 K常压条件下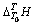 与T的关系式为
与T的关系式为
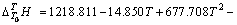
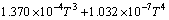 (4)
(4)
根据上述实验方法,在温度为298.15~363.15 K常压条件下溶液体系所测得的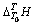 (表2~3)与温度T的关系式,减去空白时所产生的
(表2~3)与温度T的关系式,减去空白时所产生的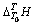 与T的关系式(方程(3)和(4)),再除以待测溶液的质量。所求得
与T的关系式(方程(3)和(4)),再除以待测溶液的质量。所求得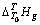 与T的关系式为
与T的关系式为
 (5)
(5)
由实验结果所得方程(5)的参数列于表4。cp与T的关系式可以通过对方程(5)求导得到,从而可求表2和3中各实验条件下铝酸钠溶液三元体系温度为T时的比定压热容
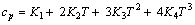 (6)
(6)
2.3 体系比定压热容与浓度及组成的关系
由于上述方法只建立了cp与T的关系式,不能求出任意浓度及组分的比定压热容,参考文献[12-13]二元水溶液体系建立模型的方式,并加以改进,试以方程(7)拟合所求得的比定压热容数据,建立能在温度298.15~363.15 K总碱度小于6 mol/kg内都能适用的铝酸钠溶液三元体系比定压热容模型。
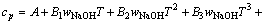
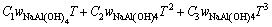 (7)
(7)
式中:T表示温度,wNaOH和wNaAl(OH)4分别是NaOH和NaAl(OH)4在铝酸钠溶液体系中的质量分数,可通过总碱度mT(mT=mNaOH+mNaAl(OH)4)及苛性比αk(αk=mT/ mNaAl(OH)4)分别由方程(8)和(9)来表示:
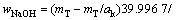
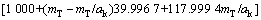 (8)
(8)

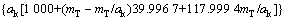 (9)
(9)
方程(7)中A、B1、B2、B3、C1、C2、C3是回归系数系数列于表5。
表4 方程(5)的回归参数
Table 4 Regression parameters for Eq.(5)
的回归参数.jpg)
表5 方程(7)的回归系数
Table 5 Regression parameters for Eq.(7)
的回归系数.jpg)
拟合方程平均偏差小于0.53%,最大不超过3%。并用拟合的方程(7)计算文献[4]在温度298.15 K条件下离子强度(I=mT=mNaOH+m NaAl(OH)4)为1~6 mol/kg及不同NaAl(OH)4(aq)含量(不包括mNaAl(OH)4=0 mol/kg)的比定压热容数据,用方程计算的比定压热容值cp,cal与文献的实验值cp,exp比较,相对平均偏差为1.4%,其中最大相对偏差为4.1%,且在浓度低于4 mol/kg,相对平均偏差为0.2%,说明所回归方程可以准确计算铝酸钠溶液离子强度1~6 mol/kg的比定压热容。
根据方程(7)所计算的铝酸钠溶液比定压热容与浓度、温度、苛性比关系如图1所示。
从图1可以看出,铝酸钠溶液的比定压热容随温度的升高而增大,同时随着总碱度的增大而减小。当总碱度mT一定时,随着溶液的αk的增大即溶液中NaAl(OH)4的浓度的减小溶液体系的比定压热容增大,这与文献[7]所研究的铝酸钠溶液体系在10 MPa的大气压强下比定压热容随温度及各组分浓度变化的规律基本一致。
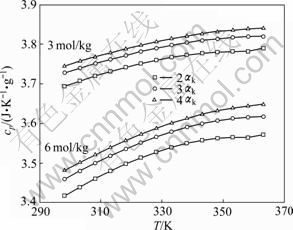
图1 不同浓度铝酸钠溶液比定压热容—温度曲线
Fig. 1 cp—T curves of sodium aluminate solution with different concentrations
2.4 铝酸钠溶液的表观摩尔比定压热容
将比定压热容数据代入下面方程[4]可求出三元体系铝酸钠溶液的表观摩尔热容
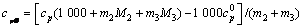 (10)
(10)
式中:cpΦ(单位为J/(K·mol))为铝酸钠溶液体系的表观摩尔热容,cp为比定压热容,m2和m3分别指该溶液体系中NaOH与NaAl(OH)4 的质量摩尔浓度,M2和M3分别指NaOH与NaAl(OH)4 的摩尔质量,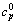 是指纯水的比定压热容,具体值见文献[11]。
是指纯水的比定压热容,具体值见文献[11]。
在铝酸钠溶液中离子强度可用总碱度mT来表示, mNaAl(OH)4浓度的变化可以用1/αk即mNaAl(OH)4/mT来表示。当温度一定时不同浓度铝酸钠溶液表观摩尔热容与1/αk关系如图2所示。
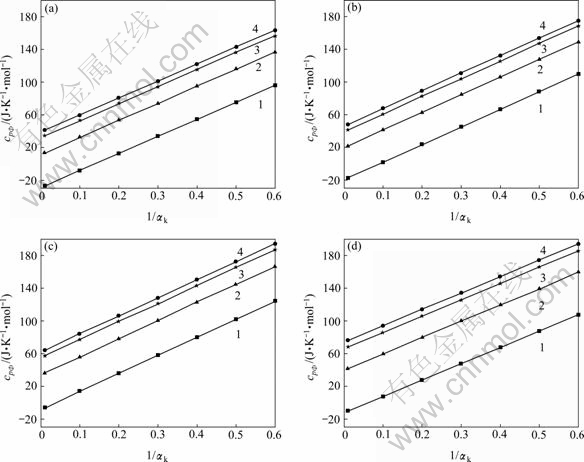
图2 不同温度下铝酸钠溶液表观摩尔热容随1/αk及离子强度I变化图
Fig. 2 Isobaric apparent molar heat capacities of sodium aluminate solution as a function of level of substitution of aluminate for 1/αk at various temperatures: (a) 298.15 K; (b) 308.15 K; (c) 333.15 K; (d) 363.15 K (constant ionic strengths: 1—1.0 mol/kg; 2—2.0 mol/kg; 3—3.0 mol/kg; 4—4.0 mol/kg)
当组成一定时,铝酸钠溶液表观摩尔热容随温度下变化规律见图3。
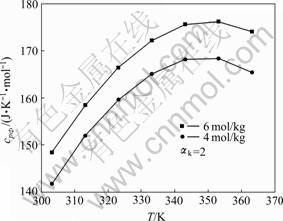
图3 铝酸钠溶液的cpΦ—T曲线
Fig. 3 cpΦ—T curves of sodium aluminate solution
从图1和2中可以知道,铝酸钠溶液在298.15~ 363.15 K温度条件下的表观摩尔热容有以下规律:当温度和αk一定时随离子强度的增大而增大;当温度一定时,随着1/αk的增大表观摩尔热容呈线性增长规律,即表观摩尔热容与mNaAl(OH)4/mT呈线性规律, 这基本符合WU[14]所引用的Young规律,这一规律使得计算该体系中纯NaAl(OH)4(aq)的表观摩尔热容成为可能;当组成一定时,温度在298.15~363.15 K下铝酸钠溶液表观摩尔热容先呈明显的增大趋势但当接近363.15 K表观摩尔热容随温度升高逐渐出现了减小趋势,根据文献[15]表明,NaOH溶液在这个温度范围内表观摩尔热容也呈先增大后在接近363.15 K时也开始逐渐减小,同时综合文献[2, 4-5, 7]可知,纯NaAl(OH)4(aq)表观摩尔热容在298.15~363.15 K也在近363.15 K时呈现出下降趋势,这与本研究所得结果基本一致。
3 结论
1) 测定了铝酸钠溶液三元体系温度在298.15~ 363.15 K,离子强度1~6 mol/kg的比定压热容,并提出了体系比定压热容与温度和浓度及苛性比的关系式,用本研究所建立的关系式计算25 ℃时离子强度1~6 mol/kg及苛性比1.9~10的比定压热容值与文献值比较结果表明:拟合的方程能较准确地计算铝酸钠溶液的比定压热容,可供工程实验和设计时使用。
2) 计算出了溶液体系的表观摩尔比定压热容,并研究表观摩尔比定压热容与溶液组成及温度的关系,表观摩尔热容与mNaAl(OH)4/mT呈线性规律, 这基本符合Young规律,这一规律使得计算该体系中纯NaAl(OH)4(aq)的表观摩尔热容成为可能。
REFERENCES
[1] 杨重愚. 氧化铝生产工艺学[M]. 修订版. 北京: 冶金工业出版社, 1993: 135.
YANG Zhong-yu. Industrial technology of aluminum oxide[M]. Revised ed. Beijing: Metallurgical Industry Press, 1993: 135.
[2] HOVEY J K, HEPLER L G, THEMAINE P R. Thermodynamics of aqueous aluminate ion: Standard partial molar heat capacities and volumes of Al(OH)4-1(aq) from 10 to 55 ℃[J]. J Phys Chem, 1988, 92(5): 1323-1332
[3] MASHOVETS V P, PUCHKOV L V, MATVEEVA R R, BARANOVA T A. Specific heats of solutions in the system Na2O-Al2O3-H2O at 150-300 ℃[J]. Tsvetnye Metally, 1969, 42(2): 60-63.
[4] MAGALHAES M C F, KONIGSBERGER E, MAY P M, HEFTER G. Heat capacities of concentrated aqueous alkaline aluminate solutions 25 ℃[J]. Journal of Chemical & Engineering Data, 2002, 47(4): 960-963.
[5] CHEN Q Y, LI X U, HEPLER G. Calorimetric study of the digestion of gibbsite, Al(OH)3(cr), and thermodynamics of aqueous aluminate ion, Al(OH)4-(aq)[J]. Canadin Journal of Chemistry, 1991, 69(11): 1685-1690.
[6] CAIANI P, CONTI G,GIANNI P, MATTEOLI E. Apparent molar heat capacity and relative enthalpy of aqueous NaOH between 323 and 523 K[J]. Journal of Solution Chemistry, 1989, 17(5): 481-497.
[7] SCHR?DLE S, K?NIGSBERGER E, MAY P M, HEFTER G. Heat capacities of aqueous sodium hydroxide/aluminate mixtures and prediction of the solubility constant of boehmite up to 300 ℃[J]. Geochimica et Cosmochimica Acta, 2010, 74(8): 2368-2379.
[8] SCHR?DLE S, K?NIGSBERGER E, MAY P M, HEFTER G. Heat capacities of aqueous solutions of sodium hydroxide and water ionization up to 300 ℃ at 10 MPa[J]. Geochimica et Cosmochimica Acta, 2008, 72(13): 3124-3138.
[9] ZHOU Jun, CHEN Qi-yuan, LI Jie, YIN Zhou-lan, ZHOU Xia, ZHANG Ping-min. Isopiestic measurement of the osmotic and activity coefficients for the NaOH-NaAl(OH)4-H2O system at 313.2 K[J]. Geochimica et Cosmochimica Acta, 2003, 67(18): 3459-3472
[10] SETARAM Co. C80Ⅱ user manual[M]. SETARAM Co: Bordeaux, 2001: 37.
[11] WANGER W, PRUSS A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use[J]. J Phys Chem, 2002, 31(2): 387-535.
[12] 田 涛, 郑丹星, 武向红, 蒋翼然. 室温离子液体[Emim]BF4及其水溶液体系的恒压热容测定[J]. 北京化工大学学报: 自然科学版, 2008, 35(3): 27-31.
TIAN Tao, ZHENG Dang-xing, WU Xiang-hong, JIANG Yi-ran. Determination the heat capacity of the ionic liquid [Emim]BF4 and its aqueous solutions[J]. Journal of Beijing University of Chemical Technology: Natural Science, 2008, 35(3): 27-31.
[13] 魏 治, 武向红, 郑丹星, 王建召, 董 丽. 离子液体[Emim]Br水溶液的比热容测量及其模型化[J]. 北京化工大学学报: 自然科学版, 2010, 37(1): 9-12.
WEI Zhi, WU Xiang-hong, ZHENG Dan-xing, WANG Jian-zhao, DONG Li. Determination of the heat capacity of aqueous solutions of [Emim]Br[J]. Journal of Beijing University of Chemical Technology: Natural Science, 2010, 37(1): 9-12.
[14] WU Y C. Young’s mixture rule and its significance[J]. J Phys Chem, 1970, 74(21): 3781-3786.
[15] ROUX A H, PERRON G, DESNOYERS J E. Heat capacities, volumes, expandabilities and compressibilities for concentrated aqueous solutions of LiOH, NaOH and KOH[J]. Can J Chem, 1984, 62(5): 878-888.
(编辑 李艳红)
<上一页 1 下一页 >