
Corrosion and wear properties of electroless Ni-P plating layer on AZ91D magnesium alloy
LI Zhong-hou(李忠厚)1, CHEN Zhi-yong(陈志勇)1, LIU Sha-sha(刘沙沙)1,
ZHENG Feng(郑 峰)2, DAI A-gan(戴阿赶)2
1. Research Institute of Surface Engineering, Taiyuan University of Technology, Taiyuan 030024, China;
2. Shanxi Seikou Institute of Magnesium Technology, Taiyuan 030002, China
Received 9 November 2007; accepted 4 July 2008
Abstract: A direct electroless Ni-P plating treatment was applied to AZ91D magnesium alloy for improving its corrosion resistance and wear resistance. Corrosion resistance of the Ni-P coatings was evaluated by potentiodynamic polarization and immersing experiments in 3.5% NaCl solution. The wear resistance of the coatings was investigated by the wear track and the mass change after ball-on-disk experiment. The results show that corrosion resistance and wear resistance of the AZ91D alloy are greatly improved after direct electroless Ni-P plating. No discoloration is noticed until 4 d of immersion in 3.5% NaCl solution. Potentiodynamic polarization experiments show that the free corrosion potential of magnesium alloy is shifted from -1 500 mV to -250 mV and passivation occurs at 1 350 mV after direct electroless plating. The friction coefficients and wear rates of Ni-P coating and Ni-P coating after tempering are 0.10-0.351, 9.038×10-3 mm3/m and 0.13-0.177, 3.056×10-4 mm3/m, respectively, at a load of 1.5 N with dry sliding. Although minor hurt on corrosion resistance was caused, significant improvement of wear resistance was obtained after tempering treatment of the coating.
Key words: magnesium alloy; direct electroless Ni-P plating; corrosion resistance; wear resistance
1 Introduction
The inherent lightness and high specific strength of magnesium alloy have brought about increasing attention on its application in automobile, motorcycle, airplane applications, etc[1-2]. However, magnesium is intrinsically highly reactive (standard potential –2.37 V) and has low hardness. Therefore, it is prone to atmospheric corrosion, and has poor wear resistance. These are actually the main obstacles for the application of magnesium alloy in practical environment [3-6].
GRAY and LUAN[7] reviewed applications of various surface technologies in surface protection of magnesium alloys. Among various surface treatments, chemical conversion, anode oxidation, painting and electroplating for magnesium alloys had been investigated intensively[8]. The electroplating is mainly conducted with cyanide and CrO3, or chromate solutions[9], hence, this treatment is harmful to environment. Electroless nickel plating on magnesium alloys is a proper protection method because it is environmental-friendly, and deposited nickel is very resistant to corrosion and abrasion. Direct electroless plating is a new processing way which needs no pre-plating layer, such as copper or zinc[10]. At present, corrosion resistance of coatings on magnesium alloy is the focus of extensive study[11-15], but literatures on wear resistance of the coatings hardly are available[5]. In this work, corrosion and wear behaviours of the nickel coating were studied in detail after direct electroless Ni-P plating on AZ91D magnesium alloy.
2 Experimental
Following the processing set-up and the parameter recommended in Ref.[10], the specimens with dimension of 15 mm×15 mm×10 mm of AZ91D magnesium alloy were processed for electroless Ni-P plating.
Some of the specimens were tempered at 400 ℃ for 1 h after electroless Ni-P plating.
The specimens of Ni-P coating and Ni-P coating after tempering were used for investigation of the corrosion and wear behaviour. Bare AZ91D and austenite stainless steel were also used in the experiment for comparison.
As the nickel coating was cathodic relatively to the substrate, pin hole penetrated through substrate can occur from the catastrophic galvanic corrosion between nickel and magnesium alloy. Hence, coating with low porosity was required for ensuring the corrosion resistance of magnesium alloy.
The porosity existing in the coating was measured by pasted paper with chemical agent, and character of the plating layer was estimated using NEOPHOT21 model optical microscope and LEO438VP model scanning electron microscope(SEM). The corrosion resistance of Ni-P coating was judged by the immersing experiments and the potentiodynamic polarization in 3.5% NaCl solution.
The test coupons were immersed in 3.5% NaCl solution, and the time taken for discoloration of the coating or formation of corrosion spots was carefully noted.
The potentiodynamic polarization experiments were conducted by PS-168 type electrochemistry test system, using a standard three-electrode configuration with saturated calomel(SCE) as a reference, a platinum electrode as counter and the sample as working electrode. A corrosion cell containing 250 mL of 3.5% NaCl solution was used, and one side of the sample was covered with resin, the opposite side with exposed area of about 1 cm2 was exposed to the solution. A polarization scan was carried out toward noble values at a rate of 1 mV/s, after allowing a steady state potential to develop.
Bare AZ91D magnesium alloy and AZ91D magnesium alloy with electroless plating were used in parallel for potentiodynamic polarization experiments. Austenite stainless steel and AZ91D magnesium alloy with electroless plating and AZ91D magnesium alloy with electroless plating and tempering treatment were also used in parallel for potentiodynamic polarization experiments.
The hardness of the coating was measured by Leco M-400-H1 microhardness tester before and after heat treatment. The wear resistance of the coating was appreciated on dry sliding condition before and after heat treatment. The WMT-1E model ball-on-disk wear test apparatus was used for wear experiment. The steel ball with diameter of 3 mm was used as a mate of the Ni-P coating for wear experiment, hardness of which is HRC 62.
The wear behavior and the wear rate of the test samples were studied by analyzing the profile of wear tracks. If the profile of a vertical section of a wear track can be measured, then the area of the profile can be calculated. The area multiplied by the mean track length represents the volume loss of the test material. Wear rate, WR, can then be found by dividing this volume loss by the total sliding distance on the disk specimen, as given by
WR=Vloss/d (1)
where Vloss is volume loss of material during the test duration, d is total sliding distance of the ball during the test period.
The cross section of the wear track is schematically shown in Fig.1. It is a part of a round with diameter of 3 mm (see the bias section in Fig.1).
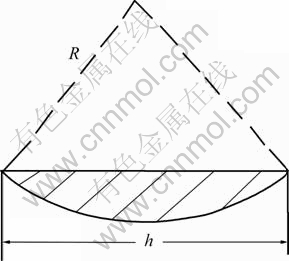
Fig.1 Sketch of cross section of wear track
Area of cross section of the wear track A can be calculated by
 (2)
(2)
The volume loss of material can be calculated through area of the profile multiplied by the average circumference of the track, then,
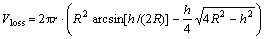 (3)
(3)
where R is radium of the mate steel ball, r is radium of the round sliding track. Width of the wear track, h, was measured from the photo of wear track after wear tests. The wear rate is calculated by Eqn.(1) and Eqn.(3).
The volume loss of the coating can also be measured from the mass change. The samples were cleaned with acetone, and mass change of the sample was measured by electronic balance with accuracy of 0.1 mg before and after wear test. In this way, Vloss can then be found by dividing this mass loss by density of the coating, as given by
Vloss=?M/D (4)
where ?M is mass loss of the sample after wear test (mg), and D is density of the coating (mg/mm3).
3 Results and discussion
Fig.1. Potentiodynamic polarization curves for bare AZ91D and AZ91D with electroless Ni plating
3.1 Corrosion resistance of coatings
Low porosity of 0.25 cm-2 in the coating was obtained after electroless plating. Figs.2 and 3 show cross section and surface morphologies of the coatings, respectively. It is obvious that the nickel coating is very compact and even and there is no defect as cavities and crevices. Thickness of the coating is about 50 μm (see Fig.2), and phosphorous content of the coating reaches 10%, which was measured by GDS (glow discharge spectrometry). Under high magnification, it can be seen that the grains consist of numerous sub-particles, and no defects exist at grain boundaries.
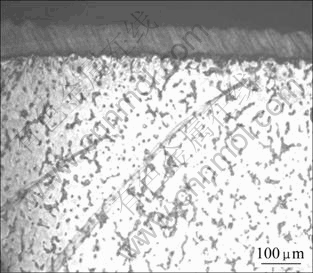
Fig.2 Microstructure of cross section after electroless Ni-P plating
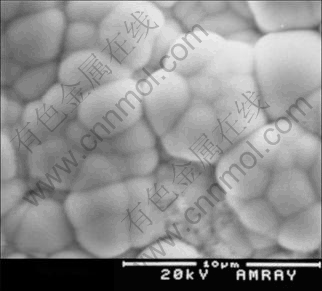
Fig.3 Surface morphology of electroless Ni-P coating under high magnification
The immersion experiment in 3.5% NaCl solution indicated that adequate corrosion resistance of the coating was obtained, because no discoloration in the coating was noticed until 4 d of immersion, and the formation of corrosion spots initiated only after 5 d of immersion.
Fig.4 shows the potentiodynamic polarization curves of bare AZ91D magnesium alloy and AZ91D magnesium alloy with electroless plating. The potentiodynamic polarization curves of austenite stainless steel, AZ91D magnesium alloy with electroless plating and AZ91D magnesium alloy with electroless plating and tempering treatment are shown in Fig.5.

Fig.4 Potentiodynamic polarization curves of Ni-P coating and bare AZ91D magnesium alloy

Fig.5 Potentiodynamic polarization curves for austenite stainless steel (a), AZ91D with electroless Ni plating (b) and AZ91D with electroless Ni plating and tempering (c)
From Fig.4, it can be observed that free corrosion potential is shifted from –1 500 mV to –250 mV after electroless Ni plating, and its polarization current is less than that of bare AZ91D under the same potential. No passivation occurs for bare AZ91D but passivation occurs at 1 350 mV for Ni-P plating. Results above show that corrosion resistance of Ni-P coating is much better than that of magnesium alloy. The even and compact Ni-P coating protects the magnesium alloy against attack of corrosion medium. So, the electroless Ni coating greatly improves the corrosion resistance of AZ91D magnesium alloy. Fig.5 shows that free corrosion potential of austenite stainless steel is –100 mV, that of AZ91D with electroless plating is –300 mV and that of AZ91D with electroless plating and tempering is –350 mV. Free corrosion current density of austenite stainless steel is less than that of Ni-P coating, and free corrosion current density of AZ91D with electroless plating is equal to that of AZ91D with electroless plating and tempering in general. It is illustrated that corrosion resistance of AZ91D with electroless plating locates between that of the austenite stainless steel and that of the AZ91D with electroless plating and tempering, and influence of tempering treatment on corrosion resistance of Ni-P coating is very mild.
3.2 Wear resistance of coating
For the convenience of depiction, the method of wear rate obtained by Eqns.(3) and (1) is called as cross section method, and that by Eqns.(4) and (1) is called as mass loss method.
In Eqn.(4), the density of Ni-P coating with 10% P is 8.19 mg/mm3 (density of Ni is 8.9 mg/mm3 and that of P is 1.82 mg/mm3).
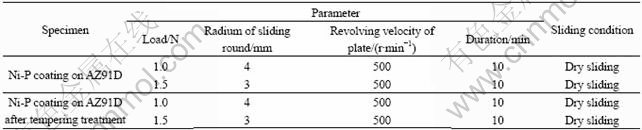
Wear test parameters used in wear test are listed in Table 1 for the Ni-P coating and the Ni-P coating after tempering treatment.
Table 1 Wear test parameters used in wear experiment
Wear rates obtained by cross section method and mass loss method are listed in Table 2 and Table 3, respectively for the Ni-P coating and the Ni-P coating after tempering treatment.
Table 2 Wear rates calculated by cross section method
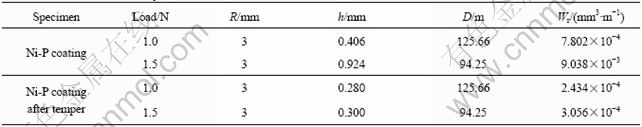
Table 3 Wear rates obtained by mass loss method

From Table 2, it can be seen that the wear rate is strongly dependent on the applied load and it increases along with increasing load irrespective of whether specimen is Ni-P coated or Ni-P coated and tempered. But then, load has more strong impact on the wear rate for Ni-P coating (load increases to 1.5 N from 1 N, wear rate magnifies almost 10 times for Ni-P coating, but for Ni-P coating and tempering, it is only 1.3 times). The wear rate of Ni-P coating after tempering is less than that of Ni-P coating observably (compare with Ni-P coating, to reduce by three times or so). As tempering treatment hardness of the Ni-P coating increases to VHN 910 from VHN 450 of plating state, as a result, wear resistance of the coating is improved greatly. Ref.[4] reported that the wear rate of bare AZ91D magnesium alloy is 2.00×10-3 mm3/m at a load of 0.745 N in condition of dry sliding, which outclasses that of 7.802×10-4 mm3/m of Ni-P coating on AZ91D at a load of 1 N in condition of dry sliding. It is obvious that electroless Ni-P plating improves obviously both the corrosion resistance and wear resistance of AZ91D magnesium alloy.
Comparing Table 2 with Table 3, it is obvious that results of wear rate from two methods are quite consistent, whether load is 1 N or 1.5 N for Ni-P coating, but for Ni-P coating after tempering, some differences of wear rate from two methods occurred whether load is 1 N or 1.5 N, which possibly arise from low accuracy of the balance.
Variations of friction coefficient with test duration are shown in Fig.6 at different loads. It can be seen that lower friction coefficient is possessed whether friction object is Ni-P coating or Ni-P coating after tempering. The friction coefficients of Ni-P coating and Ni-P coating after tempering are 0.10-0.351 and 0.13-0.177 respectively at a load of 1.5 N in condition of dry sliding. It should be noted that the amplitude of the friction coefficient oscillation of Ni-P coating after tempering is lower than that of Ni-P coating. The high hardness of Ni-P coating improves the tribo-reduction and the scrape-resistant characteristics of the coating, hence, the Ni-P coating after tempering possesses very low and very calm friction coefficient in test duration. Figs.7(a) and (b) respectively show the surface morphologies of wear tracks for the Ni-P-coating and the Ni-P coating after tempering. For Ni-P coating at a load of 1 N, medium wear regime is observed whereas severe wear regime is observed at load of 1.5 N; but for the Ni-P coating after tempering, mild wear regimes are observed whether load is 1 N or 1.5 N. Severe wear is characterized by massive surface damage and production of large metallic debris particles, which are readily identifiable during the experiment by the naked eyes. These results are quite consistent with former results of the wear rate and the friction coefficient.
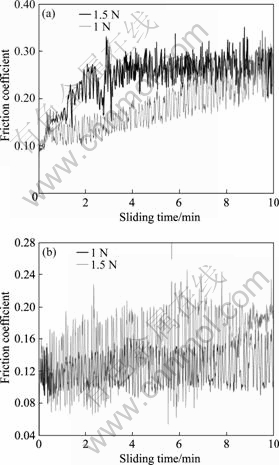
Fig.6 Variation of friction coefficient with test duration for Ni-P plating layer (a) and Ni-P plating layer after temper treatment (b) at different loads
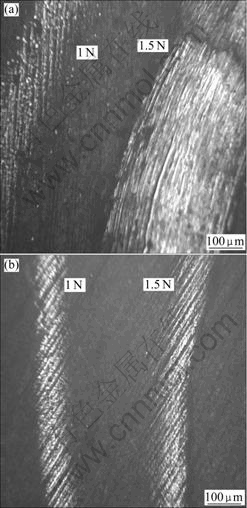
Fig.7 Surface morphologies of wear tracks: (a) Ni-P coating on AZ91D; (b) Ni-P coating on AZ91D after tempering
4 Conclusions
1) The corrosion resistance of AZ91D magnesium alloy is greatly improved by direct-electroless plating which were testified by the immersion experiment and the potentiodynamic polarization experiment in 3.5% NaCl solution.
2) Free corrosion potential shifts from –1 500 mV to –250 mV after AZ91D magnesium alloy is treated by electroless Ni plating, and polarization current decreases under the same potential for AZ91D with electroless plating compared with the bare AZ91D magnesium alloy.
3) The wear resistance of AZ91D gains a great improvement for the Ni-P coating after tempering. The friction coefficients of the Ni-P coating and the Ni-P coating after tempering are 0.10-0.351 and 0.13-0.177 respectively at a load of 1.5 N in condition of dry sliding. For the Ni-P coating after tempering, wear rate is 3.056×10-4 mm3/m at a load of 1.5 N with dry sliding, which is very low compared with that of 2.00×10-3 mm3/m at a load of 0.745 N of bare AZ91D.
References
[1] AMBAT R, ZHOU W. Electroless nickel-plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters [J]. Surface and Coating Technology, 2004, 179: 124-133.
[2] SHARMA A K, SURESH M R, BHOJRAJ H, NARAYANAMURTHY H, SAHU R P. Electroless nickel plating on magnesium alloy [J]. Metal Finishing, 1998, l96: 10-18.
[3] FROATS A, AUNE T K, HAWKE D, UNSWORTH W, HILLIS J. Corrosion of magnesium and magnesium alloys [M]. New York: ASM, 1987: 740.
[4] MAKAR G L, KRUGER J. Corrosion of magnesium [J]. International Materials Reviews, 1993, 38(3): 138-145.
[5] MEHTA D S, MASOOD S H, SONG W Q. Investigation of wear properties of magnesium and aluminium alloys for automotive application [J]. Journal of Materials Processing Technology, 2004, 155/156: 1526-1531.
[6] LIU Zhe-min, GAO Wei. The effect of substrate on the electroless nickel plating of Mg and Mg alloys [J]. Surface and Coating Technology, 2006, 200: 3553-3560.
[7] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys—Critical review [J]. Journal of Alloys and Compounds, 2002, 336: 88-113.
[8] BRUNELLI K, DABALA M, CALLIARI I, MAGRINI M. Effect of HCl pretreatment on corrosion resistance of cerium-based conversion coatings on magnesium and magnesium alloys [J]. Corrosion Science, 2005, 47: 989-1000.
[9] UMEHARA H, TAKAYA M, TERAUCHI S. Chrome-free surface treatments for magnesium alloy [J]. Surface and Coatings Technology, 2003, 169/170: 666-669.
[10] LI Zhong-hou, QU Yu-ping, ZHENG Feng, DAI A-gan. Direct electroless Ni-P plating on AZ91D magnesium alloy [J]. Trans Nonferrous Met Soc China, 2006, 16(S3): 1823-1826.
[11] DU N, PRITZKER M. Investigation of electroless plating of Ni-W-P alloy films [J]. Journal of Applied Electrochemistry, 2003, 33: 1001-1009.
[12] HUO Hong-wei, LI Ying, WANG Fu-hui. Corrosion of AZ91D magnesium alloy with a chemical conversion coating and electroless nickel layer [J]. Corrosion Science, 2004, 46: 1467-1477.
[13] BALARAJU J N, SANKARA NARAYANAN T S N, SESHADRI S K. Evaluation of the corrosion resistance of electroless Ni-P composite coatings by electrochemical impedance spectroscopy [J]. J Solid State Electrochem, 2001, 5: 334-338.
[14] GU Chang-dong, LIAN Jian-she, HE Jin-guo, JIANG Zhong-hao, JIANG Qing. High corrosion-resistance nanocrystalline Ni coating on AZ91D magnesium alloy [J]. Surface and Coating Technology, 2006, 200: 5413-5418.
[15] LI Jian-zhong, TIAN Yan-wen, HUANG Zhen-qi, ZHANG Xin. Studies of the porosity in electroless nickel deposits on magnesium alloy [J]. Applied Surface Science, 2006, 252: 2839-2846.
Foundation item: Project(2006031117-04) supported by Tackling Key Science and Technology of Shanxi Province, China; Project(07010763) supported by Academic Innovation of Taiyuan City, China
Corresponding author: LI Zhong-hou; Tel: +86-351-6010540; E-mail: lzh8085217@21cn.com
(Edited by LI Xiang-qun)