Preparation of high pure tellurium from raw tellurium containing Cu and Se by chemical method
来源期刊:中国有色金属学报(英文版)2011年第3期
论文作者:孙召明 郑雅杰
文章页码:665 - 672
关键词:碲硒化氢;砷;硝酸氧化;盐酸浸出;氢气还原
Key words:tellurium hydrogen selenide; arsenic; oxidation with nitric acid; leaching with hydrochloric acid; hydrogen reduction
摘 要:以含铜、硒的粗碲为原料,采用硝酸氧化、盐酸浸出、二氧化硫还原、氢气气氛高温处理的化学方法制备高纯碲。在浓硝酸(69%)用量为化学计量的0.96倍、液固比为4:1、反应温度为20 °C、反应时间为30 min的条件下,用硝酸氧化粗碲,粗碲中铜的去除率达到99%。粗碲氧化后用盐酸浸出,在浓盐酸用量为化学计量的1.67倍、液固比为4:1、反应温度为20 °C、反应时间为30 min的条件下,碲的浸出率为99%。浸出液中Te(IV)经二氧化硫还原,碲粉纯度达到99.95%。碲粉在反应温度为730 K的氢气中处理30 min,其纯度由99.95%上升到99.9995%。
Abstract: High pure tellurium was prepared from raw tellurium containing copper and selenium by chemical method containing oxidation with concentrated nitric acid, leaching with hydrochloric acid, reducing with sulfur dioxide and treating in hydrogen atmosphere at high temperature. Removal ratio of Cu in raw tellurium reaches 99% after raw tellurium is oxidized and leached with HNO3(69%) under the following conditions: 0.96 times stoichiometric quantity of concentrated nitric acid, 4:1 of ratio of liquid to solid, 20 °C of reaction temperature and 30 min of reaction time. Leaching ratio of Te reaches 99% after Te is leached with hydrochloric acid under the following conditions: 1.67 times stoichiometric quantity of hydrochloric acid, 4:1 of ratio of liquid to solid, 20 °C of reaction temperature and 30 min of reaction time. Tellurium powder(99.95%) is obtained when Te(IV) in leachate is reduced with sulfur dioxide. The purity of tellurium increases from 99.954% to 99.999 6% after tellurium(99.95%) is treated in hydrogen atmosphere at 723.15 K for 30 min.

SUN Zhao-ming, ZHENG Ya-jie
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 5 May 2010; accepted 14 August 2010
Abstract: High pure tellurium was prepared from raw tellurium containing copper and selenium by chemical method containing oxidation with concentrated nitric acid, leaching with hydrochloric acid, reducing with sulfur dioxide and treating in hydrogen atmosphere at high temperature. Removal ratio of Cu in raw tellurium reaches 99% after raw tellurium is oxidized and leached with HNO3(69%) under the following conditions: 0.96 times stoichiometric quantity of concentrated nitric acid, 4:1 of ratio of liquid to solid, 20 °C of reaction temperature and 30 min of reaction time. Leaching ratio of Te reaches 99% after Te is leached with hydrochloric acid under the following conditions: 1.67 times stoichiometric quantity of hydrochloric acid, 4:1 of ratio of liquid to solid, 20 °C of reaction temperature and 30 min of reaction time. Tellurium powder(99.95%) is obtained when Te(IV) in leachate is reduced with sulfur dioxide. The purity of tellurium increases from 99.954% to 99.999 6% after tellurium(99.95%) is treated in hydrogen atmosphere at 723.15 K for 30 min.
Key words: tellurium hydrogen selenide; arsenic; oxidation with nitric acid; leaching with hydrochloric acid; hydrogen reduction
1 Introduction
High pure tellurium is widely used in the fields such as solar cells[1-2], infrared detectors, imaging, optical modulators[3], fluorescent, gas sensor[4] and thermoelectric cooler[5]. Trace impurities have significant effect on physical and chemical properties of composite, and even impurities, whose contents are less than several mg/kg, play an adverse influence on properties of the electronic material[6]. High pure and ultra pure tellurium is normally prepared from tellurium with purity of 99.99% by such methods as vacuum distillation[7-9] and zone melting[10-12]. Vacuum distillation is mainly based on different boiling points of substances under vacuum condition[13]. It has been reported that it is difficult to remove Se, As, Na, K, Mg and S and other impurities by vacuum distillation[14]. Zone melting is mainly on the basis of different distribution coefficients between melted phase and solid phase. There are two intrinsic limits for zone refining. Firstly, it is difficult to separate impurities from eutectic compositions. Secondly, the infinite separation cannot be achieved since there is always a certain degree of distribution ratio in the two phases. Melting parameters, such as melting temperature, melting speed, zone length and melting times have significant influence on zone refining[10-11]. Therefore, it is difficult to prepare high pure tellurium from materials containing many impurities by zone refining. Up to the present, there are few reports on preparation of high pure tellurium from raw tellurium containing many impurities. In this work, high pure tellurium was prepared from raw tellurium, in which contents of impurities are high, by removing Cu with nitric acid, leaching Te with hydrochloric acid, reducing Te(IV) in leaching solution with sulfur dioxide, treating with hydrogen under high temperature. The technology has such advantages as simple appliance, low cost and large yield.
2 Experimental
2.1 Source and contents of raw tellurium
Raw tellurium was prepared using the procedures described by ZHENG et al[15]. In brief: copper anode slime was mixed with concentrated sulfur acid and roasted at high temperature, and Cu, Te, Se and As were leached from copper anode slime. Then copper was recovered from leachate by evaporation and cooling crystallization. The mass concentration ranges of H2SO4,Cu and Te in mother solution are 880-950, 7-10 and 3-7 g/L, respectively. Te(IV) in the mother solution was reduced by SO2 at 85 °C to yield raw tellurium when Cl- was used as catalyst. The contents, SEM image and XRD patterns of raw tellurium are shown in Table 1, Fig.1 and Fig.2, respectively. Table 1 shows that contents of Cu and Se in raw tellurium are 22.18% (mass fraction) and
Table 1 Contents of dried raw tellurium analyzed by X-ray fluorescence spectrometer (mass fraction, %)


Fig.1 SEM image of raw tellurium containing Cu and Se
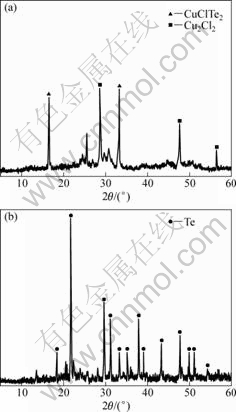
Fig.2 XRD patterns of raw tellurium: (a) Wet sample; (b) Dried sample
1.48% (mass fraction), respectively. Fig.2 shows that Cu existed in forms of Cu2Cl2 and CuClTe2, and Te existed in forms of elemental tellurium and CuClTe2. Due to SO42- and Cl- existing in solution, Pb and Ag and Ca existed in forms of PbSO4 and AgCl and CaSO4 in raw tellurium. Si existed in form of SiO2 and CaSiO3. Impurities such as PbSO4, AgCl,CaSO4, SiO2 and CaSiO3 were very difficult to dissolve in H2SO4, HNO3 and HCl.
2.2 Experimental method
Main process of preparation of high pure tellurium was as follows (Fig.3): Cu(I) and elemental Te in raw tellurium were oxidized with nitric acid(AR, 69%) to form Cu(II) and TeO2, respectively. Cu(II) was separated from tellurium by leaching with water, and TeO2 was kept in the residue. TeO2 was leached using hydrochloric acid(AR) as leaching reagent. Te(IV) in leaching solution was reduced with SO2(99.95%) to yield raw tellurium powder. Finally, raw tellurium powder was treated in hydrogen atmosphere to remove oxygen, selenium and arsenic after raw tellurium powder was oxidized, leached and reduced again. The detailed procedure of removing oxygen, selenium and arsenic was described as follows. A quart boat and a long quart tube were cleaned previously. 50 g tellurium powders were put in a quartz boat, the quartz boat was put into a long quartz tube, which was in a horizontal tube furnace; both ends of the quartz tube were sealed. The long quart tube was heated after nitrogen(99.9995%) flowed into it for 30 min. Hydrogen(99.9995%) was flowed into the long quart tube at the flow rate of 400 mL/min instead of nitrogen and reacted with part impurities while temperature in the quart tube reached a set temperature. After reaction had been processed for 30 min, heat was cut off and nitrogen was flowed again instead of H2 until temperature in the quart tube decreased to 20 °C.

Fig.3 Main process of preparation of high pure tellurium
2.3 Analysis and detection
The contents of raw tellurium and intermediate products were determined by X-ray fluorescence spectrometer(XRF, S4 pioneer) and mass concentrations of Te(IV) and Cu(II) in leaching solution were determined by inductively coupled plasma optical emission spectrometer(ICP-OES, Intrepid II XSP). The phases of products were analyzed by X-ray diffractometer (XRD, D/max-TTRIII)and the morphologies of products were observed by scanning electron microscope(SEM,JSM-6360LV). Contents of impurities in high pure tellurium were finally determined by ICP-OES after tellurium was treated with concentrated nitric acid.
3 Results and discussion
3.1 Removal of copper with concentrated nitric acid
Table 1 shows that copper is main impurity, therefore, copper must be firstly removed from raw tellurium. Standard reduction potentials of TeO2/Te, Cu2+/Cu2Cl2 and H2SeO3/Se are 0.529, 0.538, and 0.740 V[16], respectively. They are less than that of NO32-/N2O4, which is 0.803 V[16], therefore, nitric acid is used as oxidant.
Cu2Cl2 reacts with nitric acid, and the chemical reaction is as follows:
Cu2Cl2+4HNO3=CuCl2+2NO2↑+Cu(NO3)2+2H2O (1)
Cu2Cl2 forms CuCl2 and Cu(NO3)2 after it is oxidized by nitric acid. At the same time, elemental tellurium and selenium formed TeO2 and SeO2, respectively. The chemical reactions are as follows:
Te+4HNO3=TeO2+4NO2↑+2H2O (2)
Se+4HNO3=SeO2+2H2O+4NO2↑ (3)
100 g raw tellurium is put into a 2 L-beaker in each experiment, and concentrated nitric acid is slowly dropped into the beaker. Then, Cu(II) is dissolved into aqueous solution and Te is kept in the residue.
3.1.1 Effects of dosage of nitric acid on leaching rates of Cu and Te
Concentrated nitric acid is slowly dropped into the beaker at 20 °C. After reacting with Cu(I) and Te, a part of nitric acid forms NO2, and surplus nitric acid combines with Cu(II) to form Cu(NO3)2.Then leaching process has been proceed for 30 min under 6:1 of ratio of liquid to solid. The stoichiometric quantity of nitric acid was calculated according to chemical reaction (1) and reaction (2). Effects of dosage of nitric acid on leaching rates of Cu and Te are shown in Fig.4.
As shown in Fig.4, leaching rate of Cu increases with the increase of dosage of nitric acid. When the dosage of nitrate acid is 0.96 times the stoichiometric quantity, leaching rate of Cu is 98.50% and leaching rate of Te is approximately 3%. When the dosage of nitric acid increases from 0.96 times to 1.2 times, leaching rate of Te(IV) increases from 3% to 36%.
Elemental tellurium forms TeO2 while Cu2Cl2 forms CuCl2 and Cu(NO3)2 in the process of oxidation.
When the dosage of nitric acid excesses 0.96 times stoichiometric quantity, chemical reactions occur:

Fig.4 Effects of dosage of nitric acid on leaching rates of Cu and Te
CuCl2 +2HNO3=Cu(NO3)2 +2HCl (4)
TeO2 +4HCl=TeCl4 +2H2O (5)
TeO2+2HNO3=TeO(NO3)2+H2O (6)
The more the surplus dosage of nitric acid is, the higher the leaching rate of Te is. In order to remove Cu to the utmost with the minimum leaching rate of Te, an appropriate dosage of nitric acid is determined as 0.96 times stoichiometric quantity.
3.1.2 Effects of leaching temperature on leaching rates of Cu and Te
After raw tellurium has been oxidized with 0.96 times stoichiometric quantity of nitric acid, 600 mL water was used to dissolve Cu. Effects of leaching temperature on leaching rates of Cu and Te were investigated, the results are shown in Fig.5.

Fig.5 Effects of leaching temperature on leaching rates of Cu and Te
As shown in Fig.5, leaching temperature has no obvious effect on leaching rate of Cu. When Cu2Cl2 in raw tellurium forms completely CuCl2 and Cu(NO3)2, temperature has no obvious effect on solubilities of CuCl2 and Cu(NO3)2. However, leaching rate of Te increases from 2.5% to 4% when reaction temperature increases from 20 °C to 40 °C. The solubility of TeO2 increases with the increase of leaching temperature when the acidity is constant. When leaching temperature increases from 40 °C to 100 °C, leaching rate of Te decreases gradually with the increase of leaching temperature. Evaporation of nitric acid was stimulated and the concentration of nitric acid in solution decreases with the increase of leaching temperature. Therefore leaching rate of Te at higher temperature is less than that at lower temperature. The appropriate reaction temperature for leaching of Cu is 20 °C.
3.1.3 Effects of ratio of liquid to solid on leaching rates of Cu and Te
Cu is separated from tellurium at 20 °C after oxidation. Effects of ratio of liquid to solid on leaching rates of Cu and Te are shown in Fig.6.
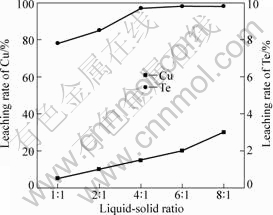
Fig.6 Effects of ratio of liquid to solid on leaching rates of Cu and Te
As shown in Fig.6, leaching rates of Cu and Te increase with the increase of ratio of liquid to solid. For instances, the leaching rate of Cu increases from 77.90% to 98.10% and the leaching rate of Te increases from 0.51% to 3.02% when the ratio of liquid to solid increases from 1:1 to 8:1, which favors the dissolution of both of CuCl2 and Cu(NO3)2 to increase the ratio of liquid to solid. Thereby, the leaching rate of Cu increases. TeO2 is slightly soluble in water, loss of tellurium increases with the increase of the ratio of liquid to solid.
Therefore, the appropriate ratio of liquid to solid is 4:1 when Cu is removed from raw tellurium.
3.1.4 Effects of leaching time on leaching rates of Cu and Te
Effects of leaching time on leaching rates of Cu and Te were investigated under the conditions of 0.96 times the stoichiometric quantity of nitric acid, 4:1 of ratio of liquid to solid and 20 °C of leaching temperature, and results are shown in Fig.7.

Fig.7 Effects of leaching time on leaching rates of Cu and Te
As shown in Fig.7, the leaching rate of Cu is 98.50% and that of Te is 2.01% when leaching has beenproceed for 30 min. It does not significantly affect their leaching rates to prolong leaching time. Therefore, the appropriate leaching time is 30 min.
From the above results, appropriate conditions of removing Cu from raw tellurium are as follows: 0.96 times stoichiometric quantity of nitric acid, 20 °C of leaching temperature, 30 min of leaching time and 4:1 of ratio of liquid to solid.
1 000 g raw tellurium powders containing copper are oxidized and leached by concentrated nitric acid under the above appropriate conditions. The volume of leachate obtained is 4.2 L, the mass concentrations of Cu and Te in the leachate are 52.3 g/L and 0.75 g/L, respectively. It can be calculated that the leaching rate of Cu is up to 99.00% and the leaching rate of Te is only 0.5% in the scale experiment in laboratory. After removing copper and drying the contents of product are listed in Table 2. The SEM image and XRD patterns of the product after removing copper with nitric acid are shown in Fig.8 and Fig.9, respectively.
As listed in Table 2, the contents of Te and Cu in the intermediate product are 77% (mass fraction) and 0.52% (mass fraction). Copper can be effectively separated from tellurium by oxidizing with concentration nitric acid. It can be known from Fig.8 and Fig.9 that the morphology of the product has an obvious change and tellurium formed TeO2 after copper has been removed.
Table 2 Contents of dried materials analyzed by XRF after removing copper with nitric acid (mass fraction, %)

Fig.8 SEM image of product after removing copper with HNO3

Fig.9 XRD pattern of raw tellurium after removing copper with HNO3
3.2 Leaching of Te with hydrochloric acid
Since TeO2 is susceptible to dissolve in hydrochloric solution, hydrochloric acid is used as leaching reagent, following reaction (5).
50 g raw tellurium, whose contents are listed in Table 2, reacts with hydrochloric acid at 20 °C for 30 min under conditions of 4:1 of rate of liquid to solid in each experiment. Effect of concentration of hydrochloric on leaching rate of Te(IV) was investigated, and the result is shown in Fig.10.

Fig.10 Effects of HCl concentration on leaching rate of Te
As shown in Fig.10, the leaching rate of Te(IV) increases significantly with the increase of the concentration of hydrochloric. When hydrochloric concentration is 10 mol/L, the leaching rate of Te(IV) reaches 99.50%. At this point, the dosage of hydrochloric acid is 1.67 times stoichiometric quantity. According to the formula c=m×w(Te)/V, it can be calculated that the concentration of Te is approximately 190 g/L. However, higher concentration of Te(IV) can result in the hydrolysis of TeCl4 and the hydrolytic reaction of TeCl4 is as follows:
TeCl4+2H2O=TeO2+4HCl (7)
In order to avoid the hydrolysis of TeCl4, concentration of hydrochloric in a solution must be kept at a certain level. Therefore, appropriate hydrochloric dosage is 1.67 times stioichiometric quantity when hydrochloric acid is used as leaching reagent.
Temperature has a significant effect on the volatility of hydrochloric. The higher the leaching temperature is, the faster the volatility rate of hydrochloric is, which will cause serious pollution of environment, corrosion of equipment, and lower utilization efficiency of hydrochloric. When the quantity of hydrochloric acid is enough, TeO2 can be completely leached at 20 °C in 30 min.
3.3 Preparation of Te powder by reducing with SO2
TeCl4 solution is obtained after tellurium has been leached by hydrochloric acid. Concentrations of Te, Cu and Se in leaching solution are 67 g/L, 0.4 g/L and 48 mg/L, respectively, after dilution.
Elemental tellurium is prepared from 10 L solution containing tellurium mentioned above after Te(IV) has been reduced at 85 °C for 8 h by SO2 whose flow rate is 60 L/h. The reduction rate of Te(IV) is 99%. The contents, SEM image and phase composition of the product after being washed and dried are shown in Table 3, Fig.11 and Fig.12, respectively.
From Table 3, it can be calculated that the purity of tellurium reaches 99.954%, and many impurities have been removed after leaching with nitric acid, dissolving with hydrochloric acid and reducing with SO2. But residual Se and As are up to 400 mg/kg and 30 mg/kg, respectively. As shown in Fig.11, the shape of product reduced is hexagonal prism. It can be known from Fig.12
Table 3 Contents of impurities in tellurium powder (mg·kg-1)


Fig.11 SEM image of Te powder after first reduction by SO2

Fig.12 XRD pattern of Te powder after first reduction by SO2
that the product is elemental tellurium.
An excess of impurities is related to residual leachate in the product after removing copper. These impurities such as Cu and As are dissolved into solution again when Te is leached. The forms of Cu and As existing in the TeCl4 solution are CuCl2, SeCl4 and H2AsO4 in the solution, respectively. Although concentration of Se(IV) in leaching solution is very lower, due to the fact that the standard reduction potential ![]() (0.74 V)[16], is more than
(0.74 V)[16], is more than ![]() , it is more easy for Se4+ to form elemental Se than Te4+ to form elemental tellurium. However, the concentration of Cu(II) is more than that of Se(IV), standard reduction potential of Cu2+/CuCl is less than that of Te4+/Te. It is difficult for Cu(II) to form Cu2Cl2. Moreover, once Cu2Cl2 is formed, it can further combine quickly with Cl- to form [CuCl2]- [16] when concentration of Cl- is very higher. This is the reason that the content of Cu in the product is less than that of Se in the intermediate product.
, it is more easy for Se4+ to form elemental Se than Te4+ to form elemental tellurium. However, the concentration of Cu(II) is more than that of Se(IV), standard reduction potential of Cu2+/CuCl is less than that of Te4+/Te. It is difficult for Cu(II) to form Cu2Cl2. Moreover, once Cu2Cl2 is formed, it can further combine quickly with Cl- to form [CuCl2]- [16] when concentration of Cl- is very higher. This is the reason that the content of Cu in the product is less than that of Se in the intermediate product.
In order to remove impurities, the first tellurium powder is treated again under appropriate reaction conditions mentioned above.
Contents, SEM image and XRD pattern of the second tellurium powder are shown in Table 4, Fig.13 and Fig.14.
As shown in Table 4, the purity of tellurium powder
Table 4 Results by ICP-OES of tellurium powder after secondary treatment

is 99.988% the contents of impurities including Cu, Pb, Ag, Cd, Ni, Sn and Mg are less than limited value required in Q/HX03—2000, but content of selenium is 100 times limited value of Se in Q/HX03—2000. It is necessary to seek for an effective way to remove selenium.
As shown in Fig.13, the shape of tellurium powder does not change, but the size of tellurium power becomes bigger than that of tellurium prepared firstly. The XRD pattern shows that crystal form has no difference from that of tellurium powder prepared firstly.
Fig.13 SEM image of Te powder after second reduction by SO2
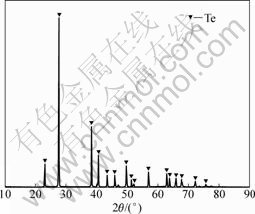
Fig.14 XRD pattern of Te powder after second reduction by SO2
3.4 Treatment of tellurium powder with secondly reduced hydrogen
In order to further decrease contents of impurities such as selenium and arsenic, tellurium powder had been treated in hydrogen for 30 min. Effects of reaction temperature on content of tellurium are considered in the present study, and the results are listed in Table 5.
Table 5 Effects of reaction temperature on content of tellurium

As listed in Table 5, contents of Se, As, Fe and Al in the tellurium gradually decrease with the increase of reaction temperature. The contents of selenium and arsenic decrease from 100 mg/kg to 0.5 mg/kg and from 10 mg/kg to 0.7 mg/kg, respectively, when the reactiontemperature increases from 523.15 K to 723.15 K.
Se exists in the forms of elemental Se in tellurium powder reduced. When reaction temperature is up to melting point of Se(490.15 K), elemental selenium is melted. The higher the reaction temperature is, the more the selenium becomes gas. When gaseous selenium meets H2, they react to product H2Se. The chemical reaction is as follows:
Se+H2=H2Se (8)
Tellurium is also possible to react with H2, chemical reaction is as follows:
Te+H2=H2Te (9)
ΔGf values of substances under different reaction temperatures are listed in Table 6 and different Gibbs free energies of chemical reactions at different temperatures are listed in Table 7.
Table 6 ΔGf values of materials at different reaction temperatures[17]

Table 7 ΔG of chemical reactions at different reaction temperatures

When reaction (8) and reaction (9) reach chemical equilibrium state, there are the following formulas:
![]() (10)
(10)
![]() (11)
(11)
According to data in Table 7 and Eqs.(10) and (11) mentioned above, the equilibrium partial pressures of both H2Se and H2Te can be calculated. Their equilibrium partial pressures at different temperatures are listed in Table 8.
Table 8 Equilibrium partial pressures of H2Se and H2Te in temperature range of 500-800 K

As shown in Table 8, equilibrium partial pressure of H2Se is very high and increases with the increase of reaction temperature. Because of the flow of hydrogen, partial pressure of H2Se in fact is far less than its equilibrium partial pressure, therefore Se can combine with H2 to form H2Se. Because the equilibrium partial pressure of H2Te in the range of 500-800 K is rather lower, elemental tellurium cannot combine with H2.
The sublimation temperature of As2O3 is 466.15 K. As2O3 firstly sublimates because of its low sublimation temperature when tellurium powder is heated. The relationship between vapor pressure of arsenic trioxide and temperature is listed in Table 9.
Table 9 Relationship between vapor pressure of arsenic trioxide and temperature[18]
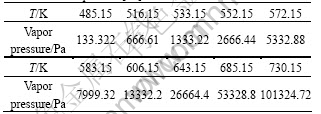
As listed in Table 9, arsenic troxide can be removed. According to Table 5, the appropriate temperature for removing As and Se is 723.15K.
4 Conclusions
1) Cu is effectively separated from raw Te materials. Leaching rate of Cu reaches 99% and leaching rate of Te is less than 3% under the following conditions of 0.96 times stoichiometric quantity of nitric acid, 4:1 of ratio of liquid to solid, 20 °C and 30 min.
2) Cu2Cl2 forms CuCl2 and Cu(NO3)2 and elemental tellurium forms TeO2 after oxidation with nitric acid.
3) TeO2 can be completely dissolved in hydrochloric acid. Leaching rate of Te reaches 99.50% under the conditions of 10 mol/L HCl, 4:1 of ratio of liquid to solid, 20 °C and 30 min.
4) The purity of tellurium power prepared from TeCl4 solution is 99.954%. After oxidation, leaching and reduction again, the purity of tellurium reaches 99.988%.
5) The purity of tellurium is 99.9996% after treatment with hydrogen at high temperature. Contents of Se and As in the tellurium are 0.5 mg/kg and 0.7 mg/kg when reaction temperature is 723.15K.
References
[1] ALESSIO B, NICOLA R, SAMAMTHA M, VITTORIO C. Polycrystalline CdTe thin films for photovoltaic applications [J]. Progress in Crystal Growth and Characterization of Materials, 2006, 52(4): 247-279.
[2] ARBAOUI A, OUTZOURHIT A, ACHARGUI N, BELLAKHDER H, AMEZIANE E L, BERNEDE J C. Effect of the zinc composition on the formation of ternary alloy Cd1-xZnxTe thin films [J]. Solar Energy Materials & Solar Cells , 2006, 90(10): 1364-1370.
[3] PAUTRAT J L. II-VI semiconductor microstructures:From physics to optoelectronics [J]. Journal of Phys, 1994, 4(12): 2413-2425.
[4] TSIULYANU D, MARIAN S, MIRON V, LIESS H D. High sensitive tellurium based NO2 gas sensor [J]. Sensors and Actuators B, 2001, 73(1): 35-39.
[5] ABDELAZIZ M M. Memory switching of germanium tellurium amorphous semiconductor [J]. Applied Surface Science, 2006, 253(4): 2059-2065.
[6] ZAIOUR A, HAGE A M, KOEBEL J M, SIFFERT P. Effective and equilibrium evaporation coefficients of some impurities in molten tellurium [J]. Materials Science and Engineering B, 1989, 3(3): 331-334.
[7] GAO Yuan, WU Hao, CHEN Hua-yue, JIANG Yu-si. Preparation of high purity tellurium by vacuum distillation technique [J]. Nonferrous Metals: Extractive Metallurgy, 2007(1): 20-22. (in Chinese)
[8] PRASAD D S, MUNIRATHNAM N R, RAO J V, PRAKASH T L. Purification of tellurium up to 5N by vacuum distillation [J]. Materials Letters, 2005, 59(16): 2035-2038.
[9] CHURBANOV M F, GERASIMENKO V V, SHIRYAEW V S. Behavior of impurity inclusions during vacuum distillation of tellurium [J]. Inorganic Materials, 2001, 37(10): 1017-1020.
[10] MOHAMAD R, KHALAD Z, ABDALLAH Z,YASSER M, MAKRAM H A. Study of segregation process of impurities in molten tellurium after one pass of three conjoint zones in zone refining [J]. Journal of Crystal Growth, 2006, 289(1): 260-268.
[11] PRASAD D S, MUNIRATHNAM N R, RAO J V, PRAKASH T L. Effect of multi-pass, zone length and translation rate on impurity segregation during zone refining of tellurium [J]. Materials Letters, 2006, 60(15): 1875-1879.
[12] KOVALEVSKY S V, SHELPAKOVA I R. High-purity zinc, cadmium, tellurium, indium and gallium: preparation and analysis [J]. Chemistry for Sustainable Development, 2000, 8: 85-87.
[13] CHENG Li-li, LI A-ling. Progress in research on extracting and refining tellurium [J]. Chinese Journal of Rare Metals, 2008, 32(1): 115-120. (in Chinese)
[14] HIRISCH H E, LIANG S C, WHITE A G. Semiconductors and semimetals [M]. Amsterdam: Academic Press, 1981, 18: 21-45.
[15] ZHENG Ya-jie, SUN Zhao-ming, WANG Bei, TENG Hao, HONG Bo. The pretreatment of copper refinery slime and the method of recovery rare elements: CN, 200810032022.0[P]. 2008-08-08. (in Chinese)
[16] CAO Xi-zhang, SONG Tian-you, WANG Xi-qiao. Inorganic chemistry [M]. Beijing: Higher Education Press, 1994. (in Chinese)
[17] BARIN G P. Thermochemical data of pure substance [M]. CHEN Nai-liang, NIU Si-tong, XU Gui-ying, et al. Beijing: Science Press, 2003. (in Chinese)
[18] LOUIE D K. Handbook of sulphuric acid manufacturing [M]. Ontario: DKL Engineering, Inc, 2005: 2-64.
孙召明,郑雅杰
中南大学 冶金科学与工程学院,长沙 410083
摘 要:以含铜、硒的粗碲为原料,采用硝酸氧化、盐酸浸出、二氧化硫还原、氢气气氛高温处理的化学方法制备高纯碲。在浓硝酸(69%)用量为化学计量的0.96倍、液固比为4:1、反应温度为20 °C、反应时间为30 min的条件下,用硝酸氧化粗碲,粗碲中铜的去除率达到99%。粗碲氧化后用盐酸浸出,在浓盐酸用量为化学计量的1.67倍、液固比为4:1、反应温度为20 °C、反应时间为30 min的条件下,碲的浸出率为99%。浸出液中Te(IV)经二氧化硫还原,碲粉纯度达到99.95%。碲粉在反应温度为730 K的氢气中处理30 min,其纯度由99.95%上升到99.9995%。
关键词:碲硒化氢;砷;硝酸氧化;盐酸浸出;氢气还原
(Edited by YANG Hua)
Corresponding author: ZHENG Ya-jie; Tel: +86-731-88836285; E-mail: zzyyjj01@yahoo.com.cn
DOI: 10.1016/S1003-6326(11)60763-2

