Thermodynamic analysis on preparation of fibrous NiO precursor powders with oxalate precipitation process
ZHAN Jing(湛 菁), ZHANG Chuan-fu(张传福), LI Tie-jing(李铁晶), WU Jian-hui(邬建辉)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: According to the principles of simultaneous equilibrium and mass balance, a series of thermodynamic equilibrium equations of Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system at ambient temperature are deduced theoretically and the logarithm concentration versus pH value(lg[Ni2+]T—pH) diagrams at different solution compositions are drawn. The results show that when pH is above 8.0, nickel ions coordinate with ammonia, the precipitation proceeds slowly accompanying with the release of nickel ions from the multi-coordinated Ni(NH3)2+n(n=1, 2, …, 6) and the morphology of NiO powder precursor is fibrous; when pH is below 8.0, nickel ion directly reacts with C2O2-4 and the morphology of NiO powder precursor is of cubic-shape. Some experiments were made to confirm the relation between the total concentration of nickel ion and pH. It is shown that the thermodynamic mathematical model is correct and the calculated values are basically accurate.
Key words: thermodynamic analysis; NiO precursor powder; fibrous shape; cubic-shape CLC number: O642.542
Document code: A
1 INTRODUCTION
The nickel oxide(abbreviated as NiO thereafter) is a versatile material, and many applications have been found in industrial fields. It is used as the key material in manufacture of battery electrode, catalyst, thermal sensitive element, gas sensitive element, functional ceramics, glass, electronic components and so on[1-6]. In these applications, the characteristics, such as chemical composition, shape, mean particle size and size distribution, are the most essential factors which influence the qualities of the final products. Therefore, it is very important to control the powder properties during the preparation process. The principal approaches available for ultrafine NiO particles production include gas-phase chemical reaction, spray pyrolysis and wet-chemical precipitation. Gas-phase chemical reaction is mainly applied by INCO Company to produce nickel and nickel oxide powders by decomposing nickel carbonyl[7]. By this process, INCO produces high-pure black NiO products with very large surface area and narrow particle size distribution in contrast to the other commercial nickel oxides. Spray pyrolysis has attracted great interest in the powder industry because of its high productive capacity and good quality of the products[8]. The nickel oxide powders obtained by this method are usually hollow, spherical, with very low apparent density. In contrast to the above two methods, the wet-chemical precipitation method[9-14] is a better preparation process for its simplicity, low-cost and good quality of the products. For wet-chemical method, there are three key procedures to prepare ultrafine powder: precipitation, drying and calcination. Usually, once the powders are formed in the precipitation, their basic characteristics, such as morphology, will be maintained. It is clear that the precipitation has great effects on the properties of the final products. So it is well worth paying more attention to study precipitation process for preparing high quality powders.
Fibrous NiO powders were also produced successfully at small scale via precipitation and thermal decomposition of the corresponding complex nickelous oxalate[13, 14]. This research is focused on the thermodynamic analysis of preparation of fibrous NiO precursor with oxalate precipitation process and applying the obtained results to guide the precipitation process.
2 THERMODYNAMIC ANALYSIS OF Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O SYSTEM
2.1 Principle of calculation method
In the system of Ni2+-C2O2-4-NH3-NH+4 -H2O, there exist not only precipitation reactions, but also coordination reactions. For the reaction of Ni2+ with C2O2-4, it can be expressed as
Ni2++C2O2-4= NiC2O4(s)
Ksp(NiC2O4(s))=[Ni2+][C2O2-4](1)
For the reaction of Ni2+ with OH-, there is a reaction:
Ni2++2OH-=Ni(OH)2(s)
Ksp(Ni(OH)2)=[Ni2+][OH-]2-(2)
Therefore, the practical concentration of free Ni2+ in the solution is deduced:
[Ni2+]=min {Ksp(NiC2O4(s))/[C2O2-4],
Ksp(Ni(OH)2)×1028-2pH}(3)
2.2 Thermodynamic data and equilibrium equations
In the system of Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O, the main chemical reactions are involved in the coordination of Ni2+ with ammonia and C2O2-4, hydrolysis of Ni2+, compounding of Ni2+ with C2O2-4, and dissociation of weak acids and alkaline. The relevant reactions are listed in Table 1. For a certain precipitation process in the solution, the variables such as temperature, pressure are usually kept constant, so in the present study, the diagrams of lg[Ni2+]T versus pH at different solution compositions are drawn for further discussion.
Table 1 Equilibrium equations and constants of Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system(T=298K)

2.3 Mathematical model of lg[Ni2+]T versus pH
Based on the principles of simultaneous equilibrium and mass balance, the mathematic models of lg[Ni2+]T versus pH at different solution compositions are deduced. Here, [Ni2+], [Ni2+]T, [N]T, and [C]T stand for the free concentration of Ni2+, the total concentration of Ni2+, the total concentration of ammonia, and the total concentration of C2O2-4, respectively.
The total concentration of coordination of Ni2+ with ammonia are expressed as [NiNH3]T in solution:
[NiNH3]T=[Ni(NH3)2+]+[Ni(NH3)2+2]+
[Ni(NH3)2+3]+[Ni(NH3)2+4]+
[Ni(NH3)2+5]+[Ni(NH3)2+6]
=[Ni2+](102.80[NH3]+105.04×
[NH3]2+106.77[NH3]3+107.96[NH3]4+
108.71[NH3]5+108.74[NH3]6)(4)
The total concentration of coordination of Ni2+ with OH- is expressed as [NiOH]T in solution:
[NiOH]T=[Ni(OH)+]+[Ni(OH)2(aq)]+
[Ni(OH)-3]
=[Ni2+](10pH-9.03+102pH-19.45+
103pH-30.70)(5)
The total concentration of coordination of Ni2+ with C2O2-4 is expressed as [NiC2O4]T in solution:
[NiC2O4]T=[Ni(C2O4)(aq)]+
[Ni(C2O4)2-2]+[Ni(C2O4)4-3]
=[Ni2+](105.3[C2O2-4]+
107.64[C2O2-4]2+108.5[C2O2-4]3)(6)
Thus, the expressions of the total concentrations of C2O2-4, NH3, Ni2+, namely CT, NT, [Ni2+]T can be obtained by
[C2O24]T=[C2O2-4]+[HC2O-4]+
[H2C2O4]+[NiC2O4]T
=[C2O2-4]{(1+105.52-2pH+
104.27-pH)+[Ni2+](105.3+2×
107.64[C2O2-4]+3×108.5[C2O2-4]2}(7)
[N]T=[NH3]+[NH+4]+[NiNH3]T
=[NH3](1+109.24-pH)+[Ni2+]·
(102.80[NH3]+2×105.04[NH3]2+
3×106.77[NH3]3+4×107.96[NH3]4+
5×108.71[NH3]5+6×108.74[NH3]6))(8)
[Ni2+]T=[Ni2+]+[NiOH]T+
[NiC2O4]T+[NiNH3]T
=[Ni2+]{1+10pH-9.03+102pH-19.45+
103pH-30.70+105.3[C2O2-4]+107.64·
[C2O2-4]2+108.5[C2O2-4]3+
102.80[NH3]+105.04[NH3]2+
106.77[NH3]3+107.96[NH3]4+
108.71[NH3]5+108.74[NH3]6}(9)
3 CALCULATED RESULTS AND DISCUSSION
The relations among the seven variables of [Ni2+]T, [Ni2+], [N]T, [NH3], [C2O2-4]T, [C2O2-4] and pH are confined by Eqns.(3)-(9). For given values of [C]T and [N]T at a certain pH, other variables can be obtained from the above mentioned simultaneous equations by the computation program complied by ourselves. The calculated results have been plotted into Figs.1-4.
Fig.1 shows the lg[Ni2+]T—pH curves for Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system(T=298K) at different total concentrations of ammonia. When pH is less than 5.0, the total concentration of nickel ions in the solution, namely [Ni2+]T decreases with pH increasing. When pH value ranges from 5.0 to 6.0, [Ni2+]T almost reaches the minimum value; in the pH range of 6.0-9.0, the total concentration of nickel ions in the solution increases sharply with pH increasing due to the strong coordination of nickel ions with ammonia. The higher the concentration of ammonia in the solution is, the higher the [Ni2+]T value in the solution is; then [Ni2+]T almost keeps constant at the maximum value when 9.0〈pH〈10.5. When pH is larger than 10.5, [Ni2+]T decreases again because nickel ammonia compound will dissociate nickel ions under such high alkaline condition and nickel ions precipitate from the solution, which leads to the decrease of total concentration of nickel ions in the solution.
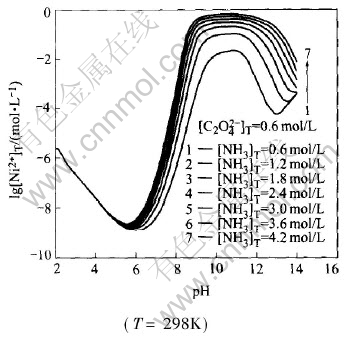
Fig.1 lg[Ni2+]T—pH curves for Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system at different total concentrations of ammonia
Fig.2 shows the lg[Ni2+]T—pH curves for Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system at different concentrations of C2O2-4(T=2981K) with [NH3]T being 3.0mol/L. The results show that the effect of the total concentration of C2O2-4 on nickel ions is similar to that of the total concentration of NH3. Due to the coordination of Ni2+ with C2O2-4, the total concentration of nickel ions becomes larger with the total concentration of C2O2-4 increasing. Therefore, the total concentration of C2O2-4 in precipitated range of pH value should be considered in experiments. In order to reduce the total concentration of nickel ions in the filtrate, the concentration of C2O2-4 is as 1.1 times as the concentration of nickel ions in solution. Thus the degree of precipitation of nickel ions in solution is almost complete.

Fig.2 lg[Ni2+]T—pH curves for Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system at different total concentrations of C2O2-4
Fig.3 and Fig.4 show the lgc—pH curves for each nickel species and nickel ammonia species in solution system(T=298K), respectively. From Fig.3, it can be found that free nickel ions are dominant in the solution when pH is less than 7.0. When 7.0〈pH〈11.5, the concentration of free nickel ions decreases with the concentration of ammonia increasing and most of the nickel ions coordinate with the ammonia as the different complex in solution. At the same time, concentration of nickel hydroxide compounds increases gradually with pH increasing. It should be noted that the total concentration of nickel species in the solution increases and reaches the maximum value due to the concentration of nickel ammonia complex. When pH>11.5, the concentration of nickel hydroxide compounds and nickel ammonia complex play important roles on the total concentration of nickel ions. Fig.4 indicates that change trends of concentration of different nickel ammonia compounds are in accordance with Ref.[16] and also further illuminate the result of the above figures.

Fig.3 lgc—pH curves for each nickel species in Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system under [NH3]T=2.0mol/L and [C2O4]2-T=0.4mol/L conditions(T=298K)
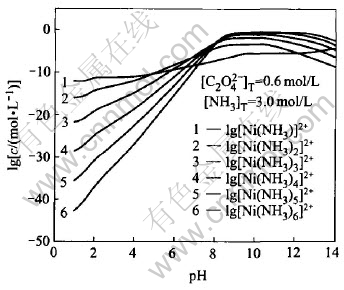
Fig.4 lgc—pH curves for each nickel species in Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system under [NH3]T=3.0mol/L and [C2O4]2-T=0.6mol/L conditions(T=298K)
4 EXPERIMENTAL RESULTS AND DISCUSSION
The detailed experimental process and influence factors on morphology of the precursors and the NiO powders were described in detail in previous papers[13, 14]. In the previous experiments, it was found that the morphology of the precipitated powder was quite different as pH was below or above 8.0. Here, the corresponding relationship between the morphology of the precipitated powders and pH values based on the experimental and theoretical results is shown in Fig.5. From Fig.5, it can be seen that when pH is below 8.0, the cubic-shaped particles are obtained; however, when pH is above 8.0, the fibre-shape particles are produced. Undoubtedly, the different solution structure leads to the formation of these two kinds of particles with different shapes. In order to obtain fibrous NiO precursor powders, the pH value range should be adjusted to 8.0-9.0 during the experimental process. If pH>9.0, nickel ions in solution can not react completely. Fig.6 shows the SEM morphologies of the typical particles prepared at pH=5.0 and pH=8.4.
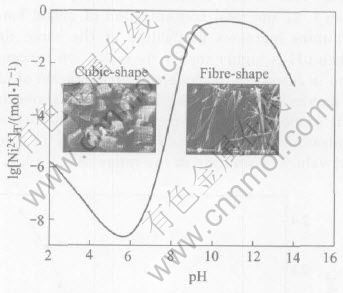
Fig.5 Relationship between morphology of precipitated particles and corresponding formation conditions in solution of Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O system

Fig.6 SEM morphologies of precipitated particles at different pH values
Fig.7 shows lg[Ni2+]T—pH curves of confirmation experiment. From Fig.7, it can be seen that the changing trend of the total concentration of metal ions is in accordance with the theoretical curves, but experimental result has slight difference from theoretically calculated values in pH range of 1.0-10.0. During the theoretical calculation, all used constants are deduced at ambient temperature while the experiments are carried out at reaction temperature of 333K. According to Fig.7, when pH is less than 7.0, [Ni2+]T decreases gradually; when pH value is about 7.0, the total concentration of nickel ions in solution reaches the minimum, namely, the precipitation rate of nickel ions is the maximum; when pH is higher than 7.5, the total concentration of nickel ions in solutions increases gradually; at the same time, when pH is higher than 9.0, nickel ion concentration in filtrate is too high and precipitation of nickel ions is incomplete. According to the above analysis of morphology of precursors and the total concentration of metal ions, it is necessary to control pH values of solution in the range of 8.0-9.0.
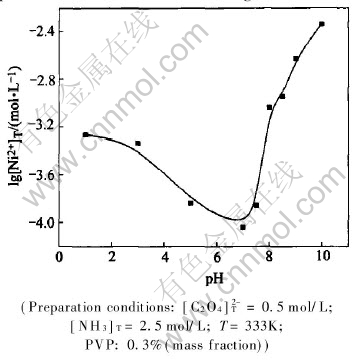
Fig.7 lg[Ni2+]T—pH curves of confirmation experiment
5 CONCLUSIONS
Based on the principles of simultaneous equilibrium and mass balance, a series of thermo-dynamic equilibrium equations of the complex system of Ni(Ⅱ)-C2O2-4-NH3-NH+4-H2O at ambient temperature are deduced theoretically and the relationship between lg[Me2+]T and pH at different solution compositions are established quantitatively with precipitation process. The theoretical calculation and experimental results show that, when pH in solution is less than 8.0, free Ni2+ is the dominant metal ion in the solution, leading to the formation of cubic-shape precursor; while pH value is larger than 8.0, most of the nickel ions coordinate with the ammonia as the complex in the solution, which leads to the formation of the fibrous precursor containing ammonia. When considering the morphology of the precursors, the optimum precipitation pH range of nickel ions in our experiments is 8.0-9.0. At the same time, confirmation experiments are carried out and the experimental results almost agree with the theoretical analysis.
REFERENCES
[1]Takehia F, Satoshi O, Hajime O, et al. Properties of NiO cathode coated with lithiated Co and Ni solid solution oxide for MCFCS [J]. Journal of Power Sources, 2001, 86: 340-346.
[2]Yoshiyui I, Yoshihiro M, Watanabe T, et al. Direct observation of the oxidation nickel in molten carbonate [J]. Journal of Particles Source,1998, 75(2): 236-243.
[3]ZHU Cheng-yi, LIU Zhong-hua, CHEN Wen. Status of preparation and application of NiO ultra-fine particles [J]. Functional Material, 1999, 30(4): 345-346.(in Chinese)
[4]XIONG Li, WANG Jin-cheng. Study on oxygen-sensitive oxide nickel material [J]. Meteorology Hydrology Ocean Apparatus, 2001(1): 17-22.(in Chinese)
[5]Hotory I, Huran J, Siciliano P, et al. The influence of preparation parameters on NiO thin film properties for gas-sensing application [J]. Sensors and Actuators B, 2001, 78: 126-132.
[6]Hotovy I, Huran J, Spiess L, et al. Preparation of nickel oxide thin films for gas sensors applications [J]. Sensors and Actuators B: Chemical, 1999, 57: 147-152.
[7]HUANG Xing-dong. Inco battery products [J].Non-Ferrous Smelting, 1998(2): 25-29. (in Chinese)
[8]Che S L, Takada K. Preparation of dense spherical Ni particles and hollow NiO particles by spraypyrolysis [J]. J Mater Sci, 1999, 34: 1313-1318.
[9]LIU Song, GU Guo-bang. Preparation of fine nickel oxide powder [J]. J South China Univ Tech, 2000, 28(3): 74-78.
[10]Matijevic E. Colloid science of ceramic powders [J]. Pure & Appl Chem, 1988, 60(10): 1479-1491.
[11]LI Ya-dong, LI Chen-wei, DUAN Xiang-feng, et al. Preparation of nanocrystalline NiO in mixed solvent [J]. Journal of China University of Science and Technology, 1997, 27(3): 346-349.
[12]ZHOU Gen-tao, LIU Shuan-huai, ZHEN Yong-fei. A study on preparation of ultrafine particles of Ni(OH)2 and NiO with different shape by precipitation transformation method [J]. Journal of Inorganic Chemistry, 1997, 13(1): 43-47.(in Chinese)
[13]ZHANG Chuan-fu, ZHAN Jing, WU Jian-hui, et al. Preparation of fibrous nickel oxide particles [J]. Trans Nonferrous Met Soc China, 2003, 13(6): 1440-1446.
[14]ZHANG Chuan-fu, ZHAN Jing, WU Jian-hui, et al. Preparation and characterization of fibrous NiO particles by thermal decomposition of nickelous complex precursors [J]. Trans Nonferrous Met Soc China, 2004, 14(4): 713-717.
[15]DEAN J A. Langes Handbook of Chemistry [M]. Beijing: Science Press, 1985. 9-17, 9-36.
[16]ZHONG Zhu-qian, MEI Guang-gui. Hydrometallurgical Processes [M]. Changsha: Central South University of Technology Press, 1988. 159-163.
(Edited by YANG Bing)
Foundation item: Project(1998053306) supported by the Doctoral Foundation of Education Ministry of China
Received date: 2004-12-10; Accepted date:2005-03-09
Correspondence: ZHAN Jing, PhD candidate; Tel: +86-731-8836037; E-mail: zhanjingandhy@163.com