
Synthesis, crystal structure and magnetic properties of
novel copper compound Cu(phen)(m-CBA)2
ZHOU Jian-liang, HUO Yan, WANG Min-min, WANG Yuan-yuan, GU Ying-ying, YI Xiao-yi, ZHANG Shou-chun
Key Laboratory of Resources Chemistry of Nonferrous Metals, School of Chemistry and Chemical Engineering,
Central South University, Changsha 410083, China
Received 10 January 2011; accepted 7 April 2011
Abstract: A novel compound Cu(phen)(m-CBA)2 was synthesized with m-chlorobenzoic acid(m-CBA), 1,10-phenanthroline(phen) and Cu(OAc)2·H2O. It was characterized by IR, UV, elemental analyses and X-ray crystallography. It crystallizes in the monoclinic crystal system with C2/c space group, a=2.9699(4) nm, b=1.15452(2) nm, c=1.5335(2) nm, β=111.118(2)°, V=4.905 1(1) nm3, Z=8, F(000)=2 328, R1=0.072 8, wR2=0.223 4 [I>2σ(I)]. Structure analysis shows that the copper center coordinates with two nitrogen atoms from one 1,10-phenanthroline molecule, two oxygen atoms from two m-chlorobenzoic acid molecules, giving a distorted squared planar coordination geometry. This novel compound shows paramagnetic interactions between copper centers.
Key words: copper (II) compound; conventional synthesis; crystal structure; magnetic properties
1 Introduction
Recently, the design and synthesis of metal aromatic carboxylic coordination compounds has become one of the hottest fields for chemists, due to their wide applications in materials, molecular electrochemistry, biochemistry and pharmaceuticals [1-3]. At present, there has been an increasing interest in the development of the structural and magnetic properties of metal aromatic carboxylic coordination compounds [4-7]. Although compounds Zn(phen)(m-CBA)2(H2O) [8], [Co(phen)2(m-CBA) (H2O)]·(m-CBA)(H2O)2 [9] and Mn(phen)(m-CBA)2 [10] with m-chlorobenzoic acid (m-CBA) and 1,10-phenanthroline (phen) ligands have been reported, copper (II) compounds with zero dimensional structural type containing m-CBA and phen ligands have not been reported. In order to obtain further information about the compound construction with aromatic carboxylic acid ligands and investigate the effect of solvent, anion and π-π interaction on the self-assembly process, a new compound Cu(phen)(m-CBA)2 with m-CBA and phen as ligands was prepared.
In this work, the synthesis and crystal structure of the compound Cu(phen)(m-CBA)2 were described. The magnetic properties of the compound Cu(phen)- (m-CBA)2 were investigated.
2 Experimental
2.1 Materials and apparatus
Copper acetate, m-chlorobenzoic acid, 1,10-phenanthroline and ethyl alcohol obtained from commercial sources were all of A.R. grade and used without further purification. IR spectra were recorded on a Perkin-Elmer 16 PC FT-IR spectrophotometer with KBr pellets in the region of 4000-400 cm-1. Elemental analyses were performed on a Perkin-Elmer 240C elemental analyzer. UV spectra were recorded on a UV-2450 ultraviolet spectrophotometer in the region of 220-400 nm. Crystal structure determination was carried out on a Bruker SMART-APEX 1000 diffractometer.
2.2 Synthesis of compound Cu(phen)(m-CBA)2
Cu(OAc)2·H2O (0.1997 g, 1 mmol), m-chlorobenzoic acid (0.3112 g, 2 mmol), 1,10-phenanthroline (0.1998 g, 1 mmol) were added into ethyl alcohol (30 mL), and the mixture solution was stirred thoroughly for 12 h at room temperature. The resulting mixture was filtered and then transparent blue hexagon crystals suited for single crystal X-ray diffraction analyses were obtained by slow evaporation at room temperature after 20 d. IR(KBr, cm-1): 1601(vs), 1558(vs), 1428(vs), 1375(vs), 1262(s), 1150(s), 881(s), 855(s), 846(vs), 768(vs), 725(vs), 664(w), 560(w), 490(w), 447(m). UV(nm): 271-305. Calcd. for C26H18O5N2CuCl2: C, 68.1; H, 3.9; N, 6.1. Found: C, 68.2; H, 3.8; N, 6.2.
2.3 X-ray crystal structure determination
X-ray measurements and data collection were carried out on a Bruker SMART-APEX 1000 area- detector diffractometer using graphite-monochromated Mo Kα radiation (λ=0.071 073 nm). The collected frames were processed with the software SAINT. The data were corrected for absorption using the program SADABS [11]. The structure was solved by direct methods and refined by full-matrix least-squares on F2 using the SHELXTL software package [12]. Non-hydrogen atoms were refined with anisotropic displacement parameters. Carbon-bonded hydrogen atoms were included in calculated positions and refined in the riding mode using SHELXL97 default parameters.
2.4 Magnetic measurements
Magnetic susceptibility data were obtained on polycrystalline samples using a Quantum Design MPMS-XL7 SQUID magnetometer. Data were recorded in field of 1.6×105 A/m while heating the sample from 1.8 to 300 K. Diamagnetic corrections were made for both the sample holder and the compound estimated from Pascal’s constants [13].
3 Results and discussion
3.1 Synthesis and characterization
The compound Cu(phen)(m-CBA)2 was obtained in high yield by treatment of m-CBA and phen with Cu(OAc)2·H2O under stirring conditions at room temperature. Compound Cu(phen)(m-CBA)2 was characterized by IR, UV, elemental analyses and X-ray diffraction. The IR spectrum of compound Cu(phen)(m-CBA)2 shows several strong sharp bands 1 601 cm-1 and 1 375 cm-1 assigned to the νas and νs stretching vibrations of coordinated carboxyl in m-CBA. The difference of νas(C=O) and νs(C=O) is 226 cm-1 and larger than 200 cm-1, indicating a monodentate coordination mode [14]. The adsorption peaks of phen (1 419, 850, 738 cm-1) shift to 1428 846 and 725 cm-1, respectively, due to its coordination with Cu(II) atom. The IR attribution of compound Cu(phen)(m-CBA)2 is consistent with the structural determination. The UV spectra of compound Cu(phen)(m-CBA)2 and ligands collected with DMSO as solvent show that m-CBA has one broad peak at about 278 nm, and phen has one strong peak at 306 nm. Compound Cu(phen)(m-CBA)2 has one broad and strong peak at about 271 nm. The shift indicates that both m-CBA and phen have coordinated with Cu(II). The formulation of compound Cu(phen)(m-CBA)2 is supported by elemental analyses.
3.2 Structural descriptions
Table 1 lists the crystallographic data and structure for compound Cu(phen)(m-CBA)2. Selected bond lengths and angles are given in Table 2. The structure of compound Cu(phen)(m-CBA)2 is shown in Fig. 1.
Table 1 Crystal data and structure for compound Cu(phen)- (m-CBA)2
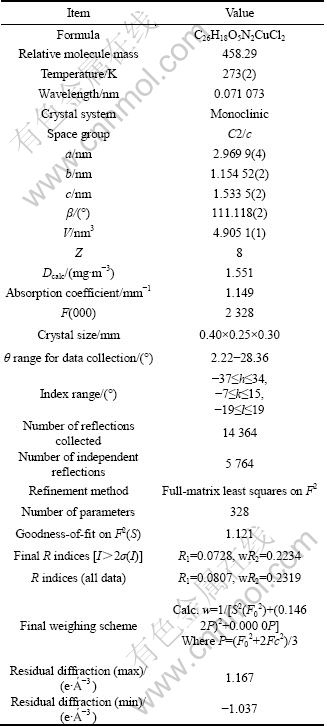
Table 2 Selected bond lengths and angles for compound Cu(phen)(m-CBA)2
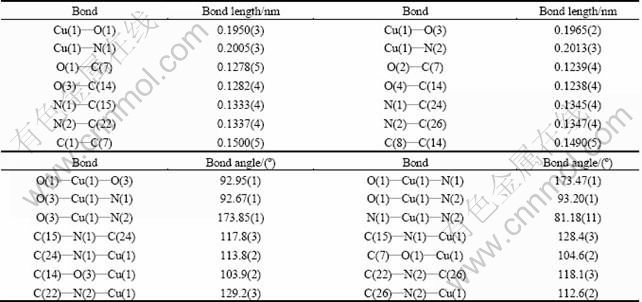
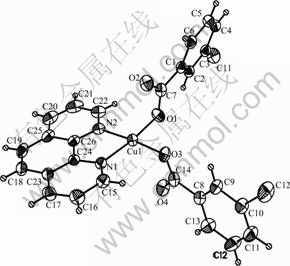
Fig. 1 Diagram of crystal structure of compound Cu(phen)- (m-CBA)2 (Thermal ellipsoids are drawn at 50% probability level)
Compound Cu(phen)(m-CBA)2 crystallizes in a monoclinic lattice with C2/c space group. As shown in Fig. 1, compound Cu(phen)(m-CBA)2 consists of one central Cu ion, one phen and two m-CBA. The copper atom has a disordered squared planar environment, which is different from compounds of Zn(phen) (m-CBA)2(H2O) [8] and [Co(phen)2(m-CBA)·(H2O)]·(m-CBA)(H2O)2 [9], but similar to Mn(phen) (m-CBA)2 [10]. The basic plane is defined by the N1, N2 atoms from phen ligand and O1, O3 atoms from m-CBA ligands. Here, bond angles N(1)—Cu(1)—N(2), O(1)—Cu(1)—N(2), O(1)—Cu(1)—O(3) and O(3)—Cu(1)—N(1) are 81.18(11)°, 93.20(12)°, 92.95(12)° and 92.67(11)°, respectively, with the sum of 360°, which suggests a planar nature of N(1), N(2), O(1) and O(3). Bond distances of Cu(1)—O(1), Cu(1)—O(3), Cu(1)—N(1) and Cu(1)—N(2) are 0.1950(3), 0.1965(2), 0.2005(3) and 0.2013(3) nm, respectively, which are shorter than those in compound [Cu2(phen)4L1]·4H2O (L1=tetra-anion of 1, 2, 4, 5-benzene tetracarboxylic acid) [15], [Cu3(btrc)2(1,10-phen)3]n (btrc=1,2,4-benzenetricar- boxylate) [16]. So, conclusion could be drawn that the central Cu(II) atom adopts a four-coordinate distorted squared planar environment. Besides, bond lengths of coordinated carboxyl are different. The bond lengths of O(1)—C(7) and O(2)—C(7) are 0.1278(5) nm and 0.1239(4) nm, respectively, with the difference of 0.0039 nm. The bond length of O(3)—C(14) (0.1282(4) nm) is also different from O(4)—C(14)(0.1238(4) nm), with the difference of 0.0044 nm, indicating a monodentate coordination mode of m-CBA.
As shown by the packing diagram (Fig. 2), weak π-π stacking interactions (centroid to centroid distance among adjacent phen planes is 0.3727 nm, while m-CBA is 0.3883 nm) are observed, which contributes to the stability of compound Cu(phen)(m-CBA)2.
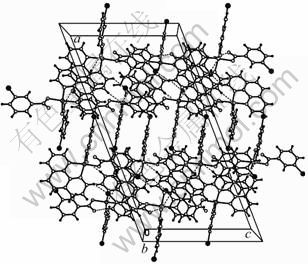
Fig. 2 Packing diagram of compound Cu(phen)(m-CBA)2 projected along b-axis direction
3.3 Magnetic properties
The temperature dependent magnetic susceptibility of compound Cu(phen)(m-CBA)2 was performed at 1.6×105 A/m in the temperature range of 1.8-300 K. Figure 3 shows the χM and χMT versus T plots for compound Cu(phen)(m-CBA)2. The χMT value is 0.15 cm3·K/mol at 300 K, which is significantly smaller than the spin of only value of 0.375 cm3·K/mol expected for a total spin S=1/2. The χMT changes slightly with decreasing temperature, which indicates paramagnetic interactions between the copper centers.
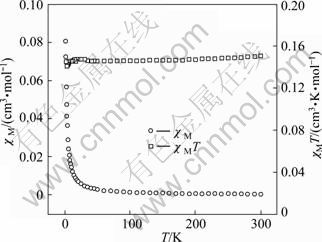
Fig. 3 χM and χMT versus T plots for compound Cu(phen) (m-CBA)2
As the temperature decreases from 300 K, χM increases slightly until 30 K, then increases quickly with temperature decreasing. This behavior also indicates paramagnetic interactions between the Cu(II) atoms. The magnetic susceptibility above 30 K obeys the Curie- Weiss law with a Weiss constant θ of -2.04 K and a Curie constant C of 0.15 (cm3·K)/mol, suggesting paramagnetic interactions between the copper centers.
Units of compound Cu(phen)(m-CBA)2 are linked by weak π-π stacking interactions, so each unit is almost independent, with the small interactions between copper centers. The paramagnetic interactions observed in compound Cu(phen)(m-CBA)2 could be mainly attributed to the sum of each Cu(II), owing to weak π-π stacking interactions between units.
4 Conclusions
1) A novel copper compound Cu(phen)(m-CBA)2 with m-chlorobenzoic acid and 1,10-phenanthroline ligands was synthesized and characterized.
2) In compound Cu(phen)(m-CBA)2, the copper atom coordinates with two nitrogen atoms from one 1,10-phenanthroline molecule, two oxygen atoms from two m-chlorobenzoic acid molecules, giving a distorted squared planar coordination geometry.
3) Paramagnetic properties exist between copper centers.
Supplementary material
CCDC 800385 contains the supplementary crystallographic data for the title compound. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/deposit, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 lEZ, UK; Fax: +44-1223-336-033; or E-mail: deposit@ccdc.cam.ac.uk.
Acknowledgements
The authors thank Professor ZHENG He-gen in Nanjing University for solving the crystal structure, and also thank Professor LIU Bin in Northwest University for collecting the magnetic susceptibility data.
References
[1] GAO Shan, GU Chang-sheng, ZHAO Hui, HUO Li-hua, ZHAO Jing-gui. Synthesis and crystal structure of 1d chain coordination polymer [Cu(3-cpoa)(phen)(H2O)]n with 3-carboxylphenoxyacetic acid ligand [J]. Chinese Journal of Inorganic Chemistry, 2004, 20(12): 1437-1440. (in Chinese)
[2] KIM J C, LOUGH A J, JO H. Syntheses and X-ray crystal structures of 14-membered tetraaza macrocyclic copper(II) complexes with polycarboxylate ligands [J]. Inorganic Chemistry Communications, 2002, 5: 616-620.
[3] WANG Xin-long, QIN Chao, WANG En-bo. Polythreading of infinite 1D chains into different structural motifs: Two poly(pseudo-rotaxane) architectures constructed by concomitant coordinative and hydrogen bonds [J]. Crystal Growth & Design, 2006, 6: 439-443.
[4] EDDAOUDI M, KIM J, O’KEEFFE M, YAGHI O M. Cu2[O-Br-C6H3(CO2)2]2(H2O)2(DMF)8(H2O)2: A framework deliberately designed to have the NbO structure type [J]. Journal of the American Chemical Society, 2002, 124: 376-377.
[5] ZHANG Jian-jun, WOJTAS L, LARSEN R W, EDDAOUDI M, ZAWOROTKO M J. Temperature and concentration control over interpenetration in a metal-organic material [J]. Journal of the American Chemical Society, 2009, 131: 17040-17041.
[6] MYRIAM R C, LESAINT C, NOGUES M, GRENECHE J M, FEREY G. Synthesis, structure, and mossbauer study of [Fe(H2O)2(C9O6H4)]H2O: A two-dimensional iron (II) trimellitate (MIL-67) [J]. Inorganic Chemistry, 2003, 42: 5669-5674.
[7] CHEN Bang-lin, OCKWIG N W, FRONCZEK F R, CONTRERAS D S, YAGHI O M. Transformation of a metal-organic framework from the NbO to PtS net [J]. Inorganic Chemistry, 2005, 44: 181-183.
[8] LUO Bing-chu, LI Chang-hong, PENG Yun-lin, KUANG Yun-fei. Hydrothermal synthesis, crystal structure and electrochemical properties of complex Zn(phen)(m-CBA)2(H2O) [J]. Chinese Journal of Structure Chemistry, 2007, 26(6): 649-653. (in Chinese)
[9] KUANG Yun-fei, LI Chang-hong, LI Wei, YANG Ying-qun. Hydrothermal synthesis, crystal structure and electrochemical properties of the complex [Co(phen)2(m-chlorobenzoic acid)2(H2O)](H2O)2 [J]. Chinese Journal of Inorganic Chemistry, 2007, 23(3): 537-540. (in Chinese)
[10] VERONICA G, MONTSERRAT C. Versatility in the coordination modes of n-chlorobenzoato ligands: Synthesis, structure and magnetic properties of three types of polynuclear MnⅡ compounds[J]. European Journal of Inorganic Chemistry, 2009, 29-30: 4471-4482.
[11] SHELDRICK G M. SADABS [M]. Germany: University of G?ttingen, 1997.
[12] SHELDRICK G M. SHELXTL-Plus V5.1 software reference manual [K]. Madison, USA: Bruker AXS Inc, 1997.
[13] KAHN O. Molecular magnetism [M]. New York: VCH, 1993.
[14] YANG Ying-qun, LI Chang-hong, LI Wei, KUANG Yun-fei. Synthesis, crystal structure and electrochemical properties of one dimensional chain coordination polymer {[Ni(4,4’-bipy)(2,4,6- TMBA)2(H2O)2](CH3OH)}n [J]. Chinese Journal of Inorganic Chemistry, 2007, 23(10): 1815-1818. (in Chinese)
[15] BARUAH A M, KARMAKAR A, BARUAH J B. Hydrolytic ring opening reactions of anhydrides for first row transition metal dicarboxylate complexes [J]. Polyhedron, 2007, 16: 4518-4524.
[16] YAN Ying, WU Chuan-de, HE Xiang, SUN Yan-qiong, LU Can-zhong. Hydrothermal synthesis of three novel multidimensional metal-organic frameworks with unusual units and mixed ligands [J]. Crystal Growth & Design, 2005, 5: 821-827.
配合物Cu(phen)(m-CBA)2的合成、晶体结构及磁性
周建良, 霍 艳, 王敏敏, 王圆圆, 古映莹, 易小艺, 张寿春
中南大学 化学化工学院,有色金属资源化学教育部重点实验室,长沙 410083
摘 要:采用间氯苯甲酸(m-CBA)、1,10-菲罗啉(phen)和醋酸铜合成一种新颖配合物Cu(phen)(m-CBA)2,并用红外光谱、紫外可见光谱、元素分析、X-射线单晶衍射对其进行表征。结果表明,该配合物为单斜晶系,空间群为C2/c,晶胞参数为a=2.9699(4) nm,b=1.15452(2) nm,c=1.5335(2) nm,β=111.118(2)°,V=4.9051(1) nm3,Z=8,F(000)=2328,R1=0.0728,wR2=0.2234 [I>2σ(I)]。结果分析表明,中心铜原子和来自1个1,10-菲罗啉分子的2个氮原子、2个间氯苯甲酸分子的2个氧原子进行配位,形成一个变形平面四边形的配位模式。该配合物中铜原子之间存在顺磁相互作用。
关键词:铜(II)配合物;常规合成;晶体结构;磁学性质
(Edited by LI Xiang-qun)
Foundation item: Project (21001118) supported by the National Natural Science Foundation of China
Corresponding author: ZHOU Jian-liang; Tel: +86-13755009868; Fax: +86-731-88879616; E-mail: zhoujl@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(11)61107-2