
Eu2+/Gd3+-codoped nanocrystalline titania catalyst and its photocatalytic activity under natural light
ZHOU Yi(周 艺)1, 2, HUANG Ke-long(黄可龙)1, ZHU Zhi-ping(朱志平)1, 2, XIA Chang-bin(夏畅斌)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Chemistry and Environment Engineering, Changsha University of Science and Technology,Changsha 410076, China
Received 15 July 2007; accepted 10 September 2007
Abstract: Eu2+/Gd3+-codoped nanocrystalline titania catalysts were prepared by a modified sol-gel method. Powder X-ray diffraction, Transmission electron microscopy and UV-Vis spectra analyse were carried out to characterize the catalysts with different Eu2+/Gd3+contents.The photocatalytic efficiency was evaluated by the photodegradation of methyl orange in an aqueous solution under natural light irradiation. It has been confirmed that Eu2+/Gd3+-codoped titania could be excited by natural light. The higher natural light activity is due to the codoping of Eu2+/Gd3+. An optimum synergetic effect was found for a mass ratio of Eu/TiO2 equal to 1.0% and Gd/TiO2 equal to 0.5%. The effects induced by Eu2+/Gd3+-codoped on the titania catalysts may be explained in terms of Gd3+→Eu2+ energy transfer to affect the photocatalysis activity of the titania catalysts.
Key words: titania catalysts; codoping; synergetic effect
1 Introduction
TiO2 photocatalyst has many advantages including innocuity, high catalytic activity, strong oxidizing power, good stability, and mild reaction condition; it has high eliminating efficiency to low concentration pollutant and gaseous pollutant, it can also operate and prepare at low cost and could be used directly in sunlight, so it is an extraordinary material to control pollution. But TiO2 photocatalyst has some disadvantages in practical usage now, for example, it has low utilizing efficiency for natural light energy, and its forbidden band is too wide that only absorbing in ultraviolet band, the combination of photoproductive electron and positive hole (express as electron/hole; e-/h+) results in low catalytic efficiency[1-2]. In order to increase the TiO2 photocatalyst capability and let it be used in practice, a great deal of efforts show that doping metal ions is an effective way[3-6]; there are many papers concerning with doping single metal ion which can increase TiO2 photocatalyst capability, but about codoping both metal ions for nanometer TiO2, special with rare earth, there are only a few reports and discussion[7]. In this work, we report a new nanometer TiO2 photocatalyst that is prepared by codoping Gd3+ and Eu2+, and has investigated its photocatalysis activity under natural light, whose target-degraded substance is methyl orange. Meanwhile the mutual action between Gd3+ and Eu2+ and the way of how to affect the photocatalysis activity of nanometer TiO2 are contrasted and analyzed; the advantages of codoping Gd3+ and Eu2+ are construed from the Eu2+-Gd3+energy transfer process, in short it is a new method to use nanometer TiO2 in practice with codoping Gd3+ and Eu2+.
2 Experimental
2.1 Catalyst preparation
Gd3+ and Eu2+ codoped nanometer TiO2 can be synthesized with sol-gel method[10], the preparing process is as following: mix the tetrabutyl titanate (CH3(CH2)3O)4Ti,CP) and absolute ethyl alcohol together according to a certain proportion, quickly drop the concentrated hydrochloric acid to above solution to adjust its pH value to approximately 3.0, then drop the mixed solution of gadolinium nitrate and europium nitrate (obtained by dissolving gadolinium oxide and europia in concentrated nitric acid ) at rate of 1/2 droplet per second; the components of mixed solution are listed in Table 1 (given as molar ratio of doping Gd3+ and Eu2+). A transparent gel can be obtained after 2 h with stopped stirring, then dry up the gel in oven at 80-100 ℃, and calcine the dried gel in muffle at 550 ℃ for 2 h, take out and mill it, the catalyzer samples given in Table 1 are obtained with different doping ratio(molar ratio of doping); The Eu2+ content is fixed at 0%, 0.1%, 0.5%, 1.0%, 1.5%, and 2.0 % in series 1-6 respectively, but Gd3+ content changes from 0% to 2.0% in each series listed in Table 1.
2.2 Characterization
Powder X-ray diffraction (SIEMENS D5000, SIEMENS, Germany), transmission electron micro- scopy (JEM-2010, JEOL Company, Japan) and UV-Vis spectra analysis (UV-2100, SHIMADZU Corporation, Japan) were carried out to characterize the catalysts with different Eu2+/Gd3+contents.
2.3 Photodegradation experiment
The photocatalysis of codoped Gd3+ and Eu2+ nanometer TiO2 were performed at homemade reaction system[8]. No pure oxygen and other substance except methyl orange were added in all of photocatalytic experiments. The natural light was used as light source and the methyl orange was employed as the target substance that could be degraded in the experiment. The concentration of methyl orange was 20 mg/L at the beginning; experiment was carried out at room temperature. Took out upper layer clear solution at a fixed period, separated by centrifugation at high speed, and then took out clear solution to measure its absorbance with spectrophotometer, and calculated the degrading rate of methyl orange. The degrading rate can be used to express the photocatalysis activity of the nanometer TiO2[9].
3 Result and discussion
3.1 XRD and TEM analysis
XRD results show that all the samples in Table 1 are anatase. The even diameter of catalyzer grain can be estimated with Scherrer formula according to full width half maximum of the furthest diffraction peak (101) in XRD pattern[10], the calculated results show that the diameter of nanometer TiO2 changes only with total doping metal mass and decreases with increasing of doping metal mass, which is the same as doping single metal ion; as the doping content of Eu2+ is 1.0%, the diameter of catalyzer grain is at its minimum value, and if the doping metal mass is increased further the diameter of catalyzer grain also increases further. In short, it is an interesting problem, further research is still performed in hand[11].
Fig.1 shows the XRD pattern of samples 20, 21, 22, and 1 in series 4 and 1.
We can make out from Fig.1 that diffraction peak of doping metal ion nanometer TiO2 widens obviously in XRD pattern, but the furthest diffraction peak (101) of pure nanometer TiO2 was sharper in XRD pattern; which is explained that doping can reduce the diameter of TiO2 grain. Because no typical diffraction peak of Gd3+ and Eu2+ appears in XRD pattern, we can deduce that doped Gd3+ and Eu2+ exists as little cluster.
Typical TEM images of codoped and undoped TiO2 samples are shown in Fig.2. In comparison with the pure
Table 1 Molar ratios of Gd3+/Eu2+ doped and degrading rate of methyl orange


Fig.1 XRD patterns of pure TiO2 sample and Eu2+/Gd3+ codoped TiO2 samples

Fig.2 TEM image of codoped Gd3+ and Eu2+ TiO2 sample (a) and pure TiO2 sample (b)
TiO2 sample (No.1), the codoped samples (No.20, 21 and 22) have relatively small particle size, and well dispersivity, indicating that the codoping can improve the particle morphology, and retarded the grain growth of TiO2 during heat-treating.
3.2 UV-Vis analysis
Fig.3 shows UV-Vis diffused reflection spectra of doped samples 19, 20, 21, 22 and 23 in series 4 and pure TiO2 sample 1. The spectra of sample 20 and sample 23 coincide together basically, spectrum of sample 21 is obvious red shift, sample 22 has good absorbance, these Gd3+ and Eu2+ four codoped samples have clearly red shift in UV-Vis spectrum and have better absorbing than only doping Eu2+ (sample 19) and pure TiO2 (sample 1). So codoping Gd3+ and Eu2+ nanometer TiO2 are favorable to absorb natural light and can increase photocatalysis activity[12]. Almost all doping of different molar ratios of Gd3+/Eu2+ can increase absorbance, but the optimum condition is Gd3+/Eu2+ equal to 0.5?1.0. The extinction capability has good coincidence relationship with photocatalysis activity, it is said that more strong extinction capability has more high photocatalysis activity.
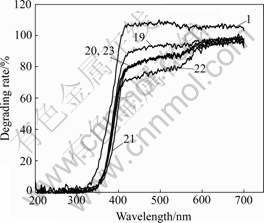
Fig.3 UV-Vis diffused reflection spectra of codoping Gd3+ and Eu2+ samples (No.20-23), doping Eu3+ sample (No.19) and pure TiO2 sample (No.1)
3.3 Photocatalysis activity of doping both Gd3+ and Eu2+ samples under natural light
According to Table 1, all samples were used in photocatalysis experiment, the experiment results are shown in Fig.4 after carried out photocatalytic reaction for 5 h under natural light.
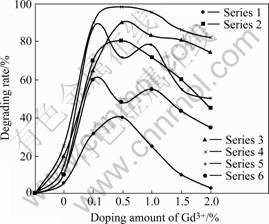
Fig.4 Photocatalysis activity comparison of codoping Gd3+ and Eu2+ samples with Eu2+ content being fixed
As shown in Fig.4 and Table 1, the codoped Gd3+ and Eu2+ nanometer TiO2 can increase photocatalysis activity at a certainty proportional ratio of Eu/Gd; as the Eu2+ content being fixed, the photocatalysis activity increases as the Gd3+ content increases within limits, but if Gd3+ content increases continually, the photocatalysis activity will decrease. It is found that in sample 21 of series 4 the highest degrading rate of methyl orange is 98% in the case of the codoping content of Eu2+ and Gd3+ at 1.0% and 0.5%, and followed by 95% for sample 22 and 90% for sample 20. So the suitable doping content for Eu2+ is 0.5%-1.0% and for Gd3+ is 0.1%-1.0%; the optimum doping content for Eu2+ is 1.0% and for Gd3+ is 0.5%.
The experiment results also show that codoping Gd3+ and Eu2+ nanometer TiO2 has higher photocatalysis activity than that of doping only single metal ion and pure nanometer TiO2, it is possible to carry out photocatalysis reaction under natural light for codoping Gd3+ and Eu2+ nanometer TiO2; simultaneously codoping Gd3+ and Eu2+ can broaden the absorbing wave range of TiO2, and the absorption band of TiO2 appeared red shift obviously as shown in UV-Vis diffused reflection spectrum (Fig.3), suggesting that less energy is needed and more long wave is produced in electron exciting process. because the width of absorption band is widened and the quantity of absorbing electrons is increased at given time under the same light source, therefore, the catalyzing efficiency is increased too.
We can explain photocatalysis essentiality of Gd3+ and Eu2+ codoped nanometer TiO2 from the theory of semiconductor defect, energy band structure and carrier transfer process[13-14]. The energy level structure is changed as doping metal ions to TiO2, the doping metal ion’s energy level not only can accept excitation electron in TiO2 excitation band, but also can absorb photon and let its electron jump to conduction band of TiO2; because the doping metal ion’s energy level locates at forbidden band, the long wave photons can be absorbed and the width of absorbance wave for TiO2 is broadened, the solar energy can be utilized effectively. At the same time, the photoproduction electron (e-) in conduction band and photoproduction positive hole (h+) in excitation band of TiO2 can be captured by the doping metal ion’s energy level, and let them separate each other. So the recombination possibility of electron and positive hole is decreased, and the carrier lifespan is prolonged, the quantity of photoproduction electron and positive hole is increased at given time and given cubage; the chance of redox which transferred by carrier at interface is increased, the photocatalysis efficiency of TiO2 is increased too.
Gd3+ and Eu2+ have an equal electron configuration, and their energy level structure are similar too; the minimum excitation energy state of Gd3+ is 6P7/2, its wavelength is at about 32 113 cm-1; Gd3+ can match well with Eu2+energy band at 4f65d. According to Dexter energy transfer theory, Gd3+ can transfer energy to Eu2+. Owing to superposition is existed in excitation spectrum of Gd3+ and Eu2+[15], the incident light can be absorbed at the same time by Gd3+ and Eu2+, the competitive absorbing is occurred between Gd3+ and Eu2+; there are positive and negative inflection to widen TiO2 absorption spectrum. The process of Gd3+ transfers energy to Eu2+ can broaden TiO2 absorption spectrum, the competitive absorbing can reduce TiO2 absorption spectrum; so there is an optimum doping molar ratio of Gd3+/Eu2+, just like as that shown in Fig.4.
4 Conclusions
1) Codoping Gd3+ and Eu2+ nanometer TiO2 can increase the photocatalysis activity at a certainty proportional ratio of Eu2+/Gd3+; the suitable doping content for Eu2+ is 0.5%-1.0% and for Gd3+ 0.1%-1.0%; the optimum doping content for Eu2+ is 1.0% and for Gd3+ 0.5%.
2) The process of Gd3+ transfers energy to Eu2+ is the primary reason for the increase of extinction capability and widening of absorb wave range for codoping Gd3+ and Eu2+ nanometer TiO2.
3) Pure TiO2 only absorbs at λ=253.7 nm, while the codoping Gd3+ and Eu2+ nanometer TiO2 extends greatly the absorb wave range, it increases extinction capability and utilization ratio of natural light. Therefore it is possible to use natural light directly as reaction light source.
References
[1] HOFFMANN M R, MARTIN S T, CHOI W. Environmental applications of semiconductor photocatalysis [J]. Chem Rev, 1995, 95: 69-74.
[2] LI F B, LI X Z, HOU M F. Photocatalytic degradation of 2-mercaptobenzothiazole in aqueous La3+-TiO2 suspension for odor control [J]. Applied Catalysis B: Environmental, 2004, 48: 185-194.
[3] JING Y A, JIA Di, LIU Yun-zhao. Effect of yttrium-doping on solar ray photocatalytic properties of titania particles to photodegradation of dyes [J]. Journal of The Chinese Ceramic Society, 2001, 29: 90-92. (in Chinese)
[4] DIANA M, TASCA M, DELIBAS M, RUSU G L. On the structural properties and optical transmittanced of TiO2 sputtered thin films [J]. Applide Surface Science, 2000, 156: 200-206.
[5] RAHMANM.M, KRISHNA K M, SOGA T, JIMBO T. Optical properties and X-ray photoelectron spectroscopic study of pure and Pb-doped TiO2 thin films [J]. Physics and Chemistry of Solids, 1999, 60: 201-210.
[6] SOBANA N, MURUGANADHAM M, SWAMINATHAN M. Nano-Ag particles doped TiO2 for efficient photodegradation of direct azo dyes [J]. Molecular Catalysis A: Chemical, 2006, 258: 124-132.
[7] HU C X, WU Y S, LUN N, SHI Y C. Influence of low temperature nitridization on the microstructure, morphology and optical properties of tin oxide nanocrystallines [J]. Materials Science and Engineering B, 2004, 110: 1-5.
[8] ZHOU Yi, XU Xie-wen, LIU Qi-cheng. Solar light photocatalysis oxidation characteristics of nano-titania particle doped with RE [J]. Journal of Central South University of Technology, 2002, 33(4): 371-373. (in Chinese)
[9] ZHANG Qing-hong, GAO Lian, GUO Jing-kun. Study about photocatalytic activity of nanometer TiO2 [J]. Journal of Inorganic Materials, 2000, 15: 556-560. (in Chinese)
[10] YIN Li-song, ZHOU Qi-fa, TANG Xin-gui, LIU Guang-min. The XRD study of nanometer TiO2 powders [J]. Journal of Functional Materials, 1999, 30: 498-511.
[11] GAO Yuan, XU An-wu, ZHU Jing-yan. Study on photocatalytic oxidation of nitrite over RE/TiO2 photocatalysts [J]. Chinese Journal of Catalysis, 2001, 22: 53-56. (in Chinese)
[12] ZANG Hua-xing, ZANG Yu-hong, XU Yong-xi, WANG Yan-guang. Phase transition and photocatalytic properties of doped terbium(Ⅲ) nanometer titanium dioxide [J]. Acta Chimica Sinica, 2003, 61: 1813-1818. (in Chinese)
[13] WEI Zi-dong, YING Fei, TAN Jun. Progress in research of TiO2 photocatalytic oxidation [J]. Chemistry Online, 2001, 64: 76-80. (in Chinese)
[14] NIU Xin-shu, LI Hong-hua, JIANG Kai. Progress in research of photocatalysis of doping Metal Ions nanometer TiO2 [J]. Electronic Components & Materials, 2004, 23: 56-60. (in Chinese)
[15] JIA Zhi-hong, LI Li, YE Ze-Ren. Gd3+→Eu2+ energy transfer in BaLiF3?Eu, Gd [J]. Chemical Journal of Chinese Universities, 2002, 23(3): 347-352. (in Chinese)
(Edited by LAI Hai-hui)
Foundation item: Project(50174024) supported by the National Natural Science Foundation of China; Project(03JJY4046) supported by the Natural Science Foundation of Hunan Province, China; Project(03C057) supported by the Scientific Research Fund of Hunan Provincial Education Department, China
Corresponding author: ZHOU Yi; Tel: +86-731-2618235; E-mail: zhouyihn@163.com