Bio-oxidation of arsenopyrite
来源期刊:中国有色金属学报(英文版)2008年第6期
论文作者:姜涛 李骞 杨永斌 李光辉 邱冠周
文章页码:1433 - 1438
Key words:arsenopyrite; electrochemistry; bio-oxidation; Acidithiobacillus ferrooxidans
Abstract: Oxidation of arsenopyrite with Acidithiobacillus ferrooxidans was studied. The electrochemical results show that arsenopyrite is firstly oxidized to As2S2 at the potential of 0.2-0.3 V (vs SHE) and As2S2 covers the electrode and retards the process continuously. While at higher potential over 0.3 V (vs SHE), As2S2 is oxidized to H3AsO3, and H3AsO3 is then oxidized to H3AsO4 at 0.8 V (vs SHE). The leaching results show that the addition of FeS2 can promote the oxidation of As3+ to As5+ and increase the activity of the bacteria. The best bio-oxidation technical parameters are the initial pH of 1.8-2.0, particle sizes less than 0.074 mm, temperature in the range of 25-30 ℃ and rotating speed of the orbital incubator of 100-160 r/min. The results provide theoretical and technological supports of bio-oxidation arsenopyrite for pretreating refractory arsenic gold ores.
基金信息:the National Natural Science Foundation of China
the National Basic Research Program of China

JIANG Tao(姜 涛), LI Qian(李 骞), YANG Yong-bin(杨永斌),
LI Guang-hui(李光辉), QIU Guan-zhou(邱冠周)
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 20 September 2008; accepted 5 November 2008
Abstract: Oxidation of arsenopyrite with Acidithiobacillus ferrooxidans was studied. The electrochemical results show that arsenopyrite is firstly oxidized to As2S2 at the potential of 0.2-0.3 V (vs SHE) and As2S2 covers the electrode and retards the process continuously. While at higher potential over 0.3 V (vs SHE), As2S2 is oxidized to H3AsO3, and H3AsO3 is then oxidized to H3AsO4 at 0.8 V (vs SHE). The leaching results show that the addition of FeS2 can promote the oxidation of As3+ to As5+ and increase the activity of the bacteria. The best bio-oxidation technical parameters are the initial pH of 1.8-2.0, particle sizes less than 0.074 mm, temperature in the range of 25-30 ℃ and rotating speed of the orbital incubator of 100-160 r/min. The results provide theoretical and technological supports of bio-oxidation arsenopyrite for pretreating refractory arsenic gold ores.
Key words: arsenopyrite; electrochemistry; bio-oxidation; Acidithiobacillus ferrooxidans
1 Introduction
At present, free milling gold ores are gradually diminishing, the low grade and refractory ores will be the main resources for extracting[1]. Arsenopyrite is the typical sulfide mineral carrying gold, and gold is generally trapped as submicroscopic particles in arsenopyrite[2]. If the Fe, As and S of the arsenopyrite are effectively oxidized by pretreating with bio-oxidation, gold will be naked and its recoveries will be increased[3].
The arsenic may be as As(Ⅲ) or As(Ⅴ) in the solution during bio-leaching processes. It is one of the important assignments for bacteria cultivation and technical study that the arsenic tolerance of the bacteria and oxidation state of the arsenic during bio-pretreatment, because As(Ⅲ) and As(Ⅴ) are all toxicity to bacteria[4]. All arsenic solubilized is in the form of As(Ⅴ) and a stable ferric arsenate product will be precipitated upon neutralization of the leach solution[5]. The reaction is as follows[6]:
4FeAsS+Fe2(SO4)3+10.5O2+3H2O→
6FeSO4+4HAsO2+H2SO4 (1)
2Fe3++As3+→2Fe2++As5+ (2)
Some researchers report that arsenic is initially dissolved as As(Ⅲ), but it is oxidized to As(Ⅴ) by contacting with water, oxygen, or ferric iron during the biooxidation process[7]. However, other researchers suggest that arsenic is dissolved as As(Ⅲ) during bio-oxidation and arsenic will remain in the trivalent oxidation state unless a stronger oxidant, such as ozone, is in presence.
It is reported that the resistance of the bacteria to As(Ⅴ) is higher than that of As(Ⅲ), which is 18 g/L and 6 g/L, respectively[8-9]. Therefore, it is very important to oxidize As(Ⅲ) to As(Ⅴ) during bioleaching.
In this work, electrochemical aspects of oxidation of arsenopyrite in the presence of Acidithiobacillus ferrooxidans were studied. At the same time, the effects of temperature, initial slurry concentration, pH value, and particle size of ores on the activity of bacteria and arsenopyrite oxidation were studied.
2 Materials and methods
2.1 Characterization of sample and bacteria
The compositions of arsenopyrite for electrochemical test are As 46.0%, Fe 34.36%, S 19.64%. The main chemical compositions and the distribution of arsenic in different mineral phases of the ore for bio- oxidation are listed in Table 1 and Table 2, respectively. It can be seen from Table 1, the grade of the iron, arsenic and sulphur is high. The main compositions of the arsenopyrite, based on the electron microprobe analyses, are As 37.25%, Fe 30.62% and S 18.37%. Arsenic is mainly as arsenopyrite phase, which is about 90.07% of the total arsenic in the sample (Table 2). In addition, in some experiments pyrite was added during the bio-oxidation process. Fe content is 41.66% according to the electron microprobe analysis results, and the particle size is under 0.074 mm.
Table 1 Main chemical compositions of sample (mass fraction, %)

Table 2 Arsenic distribution of sample (mass fraction, %)

The bacteria applied in the experiment are Acidithiobacillus ferrooxidans. The culture medium used during bacteria training and bioleaching is named 9K culture medium, and the compositions are listed in Table 3.
Table 3 Compositions of culture medium (g/L)
The electrolytic cell and all electrodes are shown elsewhere[10]. Electrolyte is 9K culture medium containing bacteria, the initial pH is 2.0 and the amount of bacteria is 109 cells/mL.
Tests were carried out in 250 mL conical flask bottle continuously shaking on an orbital incubator at required speed. At the start of each experiment, the 9K culture medium (80 mL) was injected into the reactor. A certain proportion of arsenopyrite and pyrite was added to the solution and the pH was adjusted to a given value with H2SO4. Inoculum (20 mL) was put into the bottle and the pH was readjusted. The electrical potential(φ) of the solution in the reactor was recorded periodically and liquid sample was collected. The sample was analyzed for As, total Fe and Fe2+. The reduced solution was supplied with 9K culture medium without Fe2+. The oxidizing slag was washed with distilled water, dried, and then analyzed for As after leaching.
3 Results and discussion
3.1 Anodic processes of arsenopyrite with bacteria
Under the conditions of temperature of 20 ℃, pH 2.0 and scan speed of 20 mV/min, the anodic process of arsenopyrite with bacteria is shown in Fig.1.

Fig.1 Anodic process of arsenopyrite with bacteria
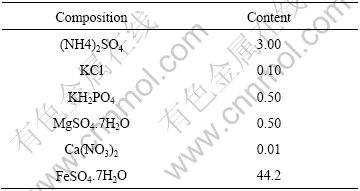
It can be seen from Fig.1 that there is a peak at the potential of 0.2-0.3 V. At the potential of 0.8V, the current density increases abruptly. The substance on the surface at 0.2-0.3 V was analyzed by XRD (Fig.2), suggesting that the arsenopyrite is firstly oxidized to As2S2, which covers on the surface and retards the further reaction that is Reaction (3). With the further rising potential, As2S2 is oxidized to arsenious acid, then to arsenic acid, and Fe2+ to Fe3+:
FeAsS→Fe2++(1/2)As2S2+2e (3)
As2S2+14H2O→2H3AsO3+2SO42-+22H++18e (4)
H3AsO3+H2O→H3AsO4+2H++2e (5)
Fe2+→Fe3++e (6)
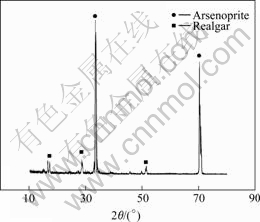
Fig.2 XRD patterns of arsenopyrite electrode surface at 0.2- 0.3 V
3.2 Effect of slurry density
The sample is crashed under 0.074 mm. The temperature in the bioreactor maintains at 30 ℃. The initial pH is 2.0, and the inoculum is 20%. The rotating speed of the orbital incubator is 140 r/min and the oxidation time is 16 d. The effect of slurry density on bio-oxidation was studied and the results are shown in Fig.3, Fig.4 and Table 4.
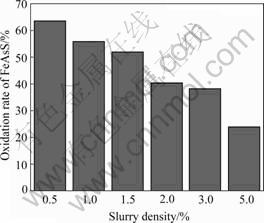
Fig.3 Effect of slurry density on oxidation
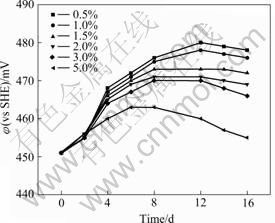
Fig.4 Effect of slurry density on potential of liquor
Table 4 Arsenic concentration at different slurry density
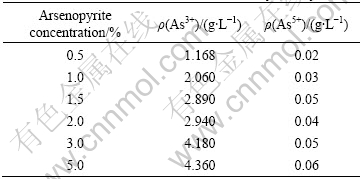
Fig.3 indicates the effect of slurry density on the dearsenization by bacteria. The oxidation of arsenopyrite quickly decreases with the slurry density increasing. When the slurry density of arsenopyrite increases to 5%, the oxidation rate reduces to only 24%.
Fig.4 shows the effect of slurry density on the electrical potential of solution. The electrical potential gradually declines at the same time with the increase of slurry density, and the electrical potential firstly increases then decreases with the bioleaching time increasing at the constant slurry density. When the slurry density is 0.5%, the electrical potential decreases after 12 d, while it is 5%, the electrical potential decreasing begins at the 6th day. The electrical potential of the solution reflects the activity of the bacteria in solution.
The electrochemical results show that when the pH is 2.0 and the electrical potential is under 800 mV, arsenic is as tervalent arsenic in the solution. The bioleaching potential is 450-480 mV (Fig.4). This result is in accordance with the results obtained by MIN[11]. These conclusions are further conformed in Table 4. Furthermore, with the increase of slurry density, the concentration of tervalent arsenic increases, detailed in Table 4, thus the activity of the bacteria reduces and E of the solution decreases.
3.3 Effect of pyrite
When two or more sulfide ores contact each other, they will form microcell based on the electrochemical theory[12-15]. Oxidation of sulfide ores by bacteria is actually an electrochemical process. When different sulfides contact each other, the one with low electrostatic potential is more reactive, and it will give its electron and be continuously corroded in acidic solution containing bacteria.
Pyrite has the highest electrostatic potential among all sulfides, and it often associates with arsenopyrite. Therefore, in this study pyrite was chosen as addition agent to investigate its effect on the bioleaching of arsenopyrite. The conditions of the test are as follows. The temperature in the bioreactor maintains at 30 ℃. The initial pH is 2.0 and inoculum is 20%. Slurry density is 1.5%. The rotating speed of the orbital incubator is 140 r/min and the experimental time is 12 d. The results are shown in Figs.5 and 6.
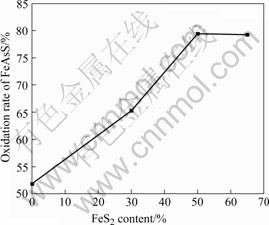
Fig.5 Effect of pyrite concentration on oxidation of arsenopyrite

Fig.6 Electrical potential at different pyrite concentrations
The arsenopyrite oxidation increases with the addition of pyrite. When the relative rate of pyrite is in the range of 50%-65%, the oxidation reaches maximumand keeps unchanged. When the concentration of pyrite is 65%, the oxidation reaches 79.23% after being oxidized for 12 d. The result increases by 15.83% compared with pure arsenopyrite oxidized after 16 d, of which the concentration of arsenopyrite is 0.5%. It is assumed that adding pyrite will form microcell and comparatively active arsenopyrite will be first oxidized. Meanwhile, As(Ⅲ) is converted to As(Ⅴ) under the combined action of pyrite and Fe3+. Consequently, the bacteria keep high activity, which can be confirmed from Fig.6. The electrical potential of solution ascends quickly and does not decrease. It is found that the concentration of bacteria keeps higher in the course of study.
3.4 Effect of initial pH
The temperature maintains at 30 ℃. Inoculum is 20%. The rotating speed of the orbital incubator is 140 r/min. The slurry density is 1.5%. The concentration of pyrite is 30% and the leaching time is 12 d. Under these conditions, the effect of initial pH of the solution on arsenopyrite oxidation rate was studied. The results are shown in Fig.7.
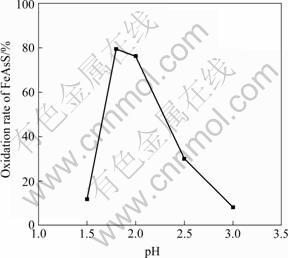
Fig.7 Effect of initial pH on oxidation of arsenopyrite
The arsenopyrite oxidation firstly rises then decreases with pH increasing. When pH is 1.8, the oxidation rate reaches its maximum as 79.58%. When pH is 1.5 and 3.0, the oxidation rate is only about 10%. It is found that when pH is 1.5, electrical potential is low and not changeable during the whole bioleaching process, which indicates that the bacteria are difficult to survive. When pH is 3.0, although the bacteria are alive, the color of the residue is yellow, and the yellow substance is arrosite (show in Fig.8), it covers on the surface of arsenopyrite and blocks bacteria from further oxidizing. When pH is 1.8, electrical potential of solution is higher and Fe3+ is difficult to deposit, which are advantageous for transforming of As(Ⅲ) to As(Ⅴ), consequently, the oxidation of arsenopyrite is higher.

Fig.8 XRD pattern of residue at pH 3.0
The temperature maintains at 30 ℃. The initial pH is 2.0 and inoculum is 20%. The rotating speed of the orbital incubator is 140 r/min. The slurry density is 1.5%. The concentration of pyrite is 30% and the leaching time is 12 d. Under these conditions, the effect of particle size on arsenopyrite oxidation rate was studied. The results are shown in Table 5.
Table 5 Effect of particle size on arsenopyrite oxidation
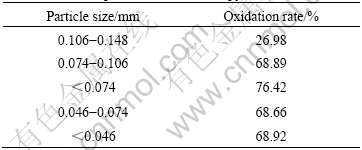
The arsenopyrite oxidation rate firstly rises then decreases with particle sizes increasing. When the particle size is under 0.074 mm, the oxidation rate reaches maximum. Lager particle size leads to a decrease of bacteria adsorbed on the surface. However, when the size is too small, the probability of particle impacting with each other increases, as well as with bacteria, it causes cellular structure of bacteria to be damaged and affects the growth of bacteria. Furthermore, the viscosity of slurry is increased and the retardation of the air is enhanced if the size is too small, which leads to decrease the oxidizing ability of bacteria.
3.6 Effect of temperature
The inoculum is 20%, and the initial pH is 2.0. The rotating speed of the orbital incubator is 140 r/min. The slurry density is 1.5%. The concentration of pyrite is 30% and the leaching time is 12 d. Under these conditions, the effect of different temperatures in the bioreactor on arsenopyrite oxidation rate was studied. The results are shown in Fig.9.

Fig.9 Effect of temperature on oxidation of arsenopyrite
Thiobacillus ferrooxidans survives at temperature of 25-35 ℃. The oxidation ratio decreases if temperature is over 35 ℃. It is found that the oxidation speed of ferrous ion and E of the solution are the highest at 30 ℃, which is advantageous for the transformation of As(Ⅲ) to As(Ⅴ).
The temperature maintains at 30 ℃, and the initial pH is 2.0. Inoculum is 20%. Slurry density is 1.5%. The concentration of pyrite is 30% and the leaching time is 12 d. Under these conditions, the effect of rotating speed on arsenopyrite oxidation rate was studied. The results are shown in Fig.10.
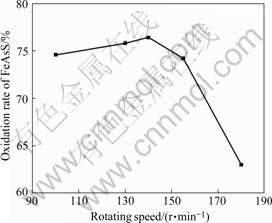
Fig.10 Effect of rotating speed on oxidation of arsenopyrite
It is disadvantageous for bacteria to oxidize arsenopyrite if the rotating speed is too high or too low. Bacteria obtain energy to autotrophic nutrition by cytoplasm between cellwall and cytoplasmic membrane. When the rotating speed is higher, the shearing intensity is stronger. If the intensity is beyond the tolerance limit of the cellwall, the bacteria will be damaged because its cellwall is very thin and the growth of bacteria will be slower. In reverse, when the rotating speed is slower, the oxygen content in the solution is low, which leads to a low bacteria oxidizing ability.
4 Conclusions
1) Arsenopyrite is firstly oxidized to As2S2 at the potential of 0.2-0.3 V (vs SHE), and As2S2 is then oxidized to H3AsO3 at higher potential. When the potential increases to 0.8 V (vs SHE), H3AsO3 is oxidized to H3AsO4.
2) The pure arsenopyrite oxidation is low, and arsenic occurs mainly as tervalent arsenic in the solution. The maximum resistance of the bacteria to As(Ⅲ) is 4.36 g/L.
3) Arsenopyrite is first oxidized to As(Ⅲ), and then oxidized to As(Ⅴ) under the combined action of pyrite and Fe3+, and the oxidation rate increases.
4) The oxidation rate is higher under the conditions of the particles size under 0.074 mm, temperature of 25-30 ℃ and pH 1.8-2.0.
References
[1] GONZALEZ R, GENTINA J C, ACEVEDO F. Continuous biooxidation of a refractory gold concentrate [C]// Biohydro- metallurgy and the Environment Toward the Mining of 21st Century (Part A). Amsterdam: Elsevier, 1999: 309-317.
[2] GONZALEZ R, GENTINA J C, ACEVEDO F. Biooxidation of a gold concentrate in a continuous stirred tank reactor: Mathematical model and optimal configuration [J]. Biochemical Engineering Journal, 2004, 19: 33-42.
[3] WANG Yu-min, JI Shou-yuan, CHEN Ke-rong. New discussion of submicroscopic micrographic gold in pyrite and arsenopyrite [J]. Mineral Journal, 1994, 14(1): 83-87.
[4] ZHANG Zhen-ru, WANG Qing-duo, LIAO Fen-xian. The investigation of submicroscopic gold in arsenopyrite [J]. Journal of Guilin Metallurgy and Geological Academy, 1991, 11(2): 150-153.
[5] ZHANG Yong-zhu, LU Yi-yuan, ZHANG Chuan-fu. Biooxidation and cyanidation of arsenic bearing gold ore [J]. The Chinese Journal of Nonferrous Metals, 1994, 4(2): 17-22. (in Chinese)
[6] EDWARDS K J, HU B. A new look at microbial leaching patterns on sulfide minerals [J]. Microbiology Ecology, 2001, 34: 197-206.
[7] BREED A W, GLATZ A, HANSFORD G S. The effect of As3+ and As5+on the batch bioleaching of a pyrite-arsenopyrit concentrate [J]. Minerals Engineering, 1996, 9(12): 1235-1252.
[8] TOMKINS A G, FROST B R, DAVID R M. Arsenopyrite melting during metamorphism of sulfide ore deposits [J]. Canadian Mineralogist, 2006, 44(5): 1045-1062.
[9] MARLA T, PETER D G, SHELLY M. Multiple sets of arsenic resistance genes are present within highly arsenic-resistant industrial strains of the biomining bacterium, Acidithiobacillus caldus [C]// International Congress Series. 2004: 165-172.
[10] LI Qian, YANG Yong-bin, JIANG Tao, QIU Guan-zhou. Electrochemical research of oxidation of arsenopyrite in acidic media [J]. The Chinese Journal of Nonferrous Metals, 2006, 16(11): 1971-1975. (in Chinese)
[11] MIN Xiao-bo. The investigation of base theory and technology on bioleaching of arsenic bearing gold ore [D]. Changsha: Central South University, 2000: 33-39.
[12] YANG Hong-ying, YANG Li, WEI Xu-jun. Mechanism on bio-oxidation of arsenopyrite with Thiobacillus ferrooxidans strain SH-T [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(2): 323-327. (in Chinese)
[13] YANG Hong-ying, YANG Li, ZHAO Yu-shan. Active alignment of sulphide minerals biooxidation by Thiobacillus ferrooxidans [J]. Nonferrous Metals, 1998, 54(2) :42-45.
[14] LI Xiu-yan, ZHOU Jian-min, WEI De-zhou. Function of ferric irons on the bioleaching of sulfide minerals [J]. Journal of Northeastern University, 2001, 22(3): 291-294. (in Chinese)
[15] GUSTAVO U, REYES V E, VELOZ M A, GONZALEZ I, CRUZ J. Pyrite-arsenopyrite galvanic interaction and electrochemical reactivity [J]. Journal of Physical Chemistry, 2008, 112(28): 10453-10461.
Foundation item: Project(50321402) supported by the National Natural Science Foundation of China; Project(2004CB619204) supported by the National Basic Research Program of China
Corresponding authors: JIANG Tao; Tel: +86-731-8877656; E-mail: jiangtao@mail.csu.edu.cn

