
Effects of rapid quenching on structure and electrochemical characteristics of La0.5Ce0.2Mg0.3Co0.4Ni2.6-xMnx (x=0-0.4) electrode alloys
ZHANG Yang-huan(张羊换)1, 2, ZHAO Dong-liang(赵栋梁)1, DONG Xiao-ping(董小平)1, 3,
QI Yan(祁 焱)1, GUO Shi-hai(郭世海)1, WANG Xin-lin(王新林)1
1. Department of Functional Material Research, Central Iron and Steel Research Institute, Beijing 100081, China;
2. School of Material, Inner Mongolia University of Science and Technology, Baotou 014010, China;
3. School of Materials Science and Engineering, University of Science and Technology Beijing,
Beijing 100083, China
Received 5 March 2008; accepted 18 June 2008
Abstract: The La-Mg-Ni system PuNi3-type La0.5Ce0.2Mg0.3Co0.4Ni2.6-xMnx (x=0, 0.1, 0.2, 0.3, 0.4) hydrogen storage alloys were prepared by casting and rapid quenching. The effects of the rapid quenching on the structure and electrochemical characteristics of the alloys were studied. The results obtained by XRD, SEM and TEM indicate that the as-cast and quenched alloys mainly consist of two major phases, (La,Mg)Ni3 and LaNi5, as well as a residual phase LaNi. The rapid quenching does not exert an obvious influence on the phase composition of the alloys, but it leads to an increase of the LaNi5 phase and a decrease of the (La, Mg)Ni3 phase. The as-quenched alloys have a nano-crystalline structure, and the grain sizes of the alloys are in the range of 20-30 nm. The results by the electrochemical measurements indicate that both the discharge capacity and the high rate discharge(HRD) ability of the alloy first increase and then decrease with the variety of quenching rate and obtain the maximum values at the special quenching rate which is changeable with the variety of Mn content. The rapid quenching significantly improves the cycle stabilities of the alloys, but it slightly impairs the activation capabilities of the alloys.
Key words: La-Mg-Ni system electrode alloy; rapid quenching; structure; electrochemical performance
1 Introduction
Intermetallic compounds for reversible hydrogen absorption/desorption have been the subject of extensive research for about 30 years. Consequently, a series of metal hydride electrode materials have been discovered, including the rare-earth-based AB5-type alloys[1], the AB2-type Laves phase alloys[2], the V-based solid solution alloys[3] and the Mg-based alloys[4], and the output of the small size Ni-MH cells has rapidly grown and gained a good share of the rechargeable battery market since the commercialization of the small size Ni-MH cells in 1990. Recently, Europe community and major developed countries in the world issued in succession decree to forbidding the Ni-Cd power battery to be continually used, which provides a golden opportunity for the development of the Ni-MH battery. However, none of the available electrode alloys above- mentioned can meet the specification of the power battery owing to the limitation of their properties, such as the low discharge capacity of the AB5-type electrode alloy, the poor activation capability of the AB2-type Laves phase as well as V-based solid solution electrode alloys and the poor cycle stability of the Mg-based electrode alloy. Therefore, the attention in this area has been paid to finding new type electrode alloys with higher capacity and longer cycle life. Recently, R-Mg-Ni-based (where R is a rare earth or Y, Ca) PuNi3-type alloys were reported, which were considered to be one of the most promising candidates owing to their high discharge capacities (360-410 mA?h/g) and low production costs in spite of their poor cycle stabilities[5-13]. KADIR et al[5-7] revealed that the alloy had a PuNi3-type rhombohedral structure, and reported that the hydrogen storage capacity of (Y0.5Ca0.5)(MgCa)Ni9 alloy was 1.98% (mass fraction) at 3.3 MPa and 263 K for the gas-solid reaction. KOHNO et al[8] found that the La5Mg2Ni23-type electrode alloy La0.7Mg0.3Ni2.8Co0.5 had a capacity of 410 mA?h/g, and good cycle stability during 30 charge-discharge cycles. LEI et al[9] investigated the electrochemical properties of LaxMg3-xNi9 (x=1.0-2.0) alloys and found that the alloy (x=2) exhibited the highest discharge capacity of 403 mA?h/g. After the structures and electrochemical performances of the La0.67Mg0.33Ni3.0-xCox (x=0-0.75) alloys were investigated, LUO et al[10] reported that the alloy (x=0.5) obtained a maximum discharge capacity of 404.47 mA?h/g, and the addition of Co significantly improved the cycle stabilities of the alloys. TANG et al[11] investigated the influence of Al substitution for Co on the structure and hydrogen storage characteristics of the Ml0.8Mg0.2Ni3.2Co0.6-xAlx (x=0-0.6) hydrogen storage alloys, confirming that the Al substitution significantly enhanced the stability of the alloy hydrides. ZHANG et al[12] researched the crystal structures and the electrochemical characteristics of the La0.7Mg0.3- Ni3.5-x(Al0.5Mo0.5)x (x=0-0.8) alloys and obtained the maximum discharge capacity of 397.6 mA?h/g (x=0.6). PAN et al[13-14] researched the influence of element addition and substitution on the structures and electrochemical behaviours of the alloys, indicating that the addition and substitution of elements Al, Cu, Fe, Mn, Co and Zr significantly improved the electrochemical performances of the alloys. It is well known that the preparation technology is vital for improving the performances of the alloys. Our previous works have confirmed that rapid quenching is a very effective method for improving the electrochemical characteristics of the electrode alloys, especially their cycle stabilities[15-17]. Therefore, it is expected that the combination of an optimized amount of Mn substitution for Ni with a proper rapid quenching technique may produce an alloy with high discharge capacity and good cycling stability. Aiming on this purpose, the effects of the rapid quenching on the structures and electrochemical characteristics of the La0.5Ce0.2Mg0.3- Co0.4Ni2.6-xMnx (x=0-0.4) electrode alloys were systematically investigated.
2 Experimental
The purity of La, Ce, Ni, Co, Mg and Mn used as the raw materials was at least 99.8%. The experimental alloys were melted by a vacuum induction furnace. High purity helium with a pressure of 0.04 MPa was used as a protecting atmosphere for effectively preventing the volatilization of magnesium during melting. A cast ingot was thus obtained by pouring the melt into a copper mould cooled by water. Part of the as-cast alloys was re-melted and quenched by melt-spinning with a rotating copper roller in a helium atmosphere. The quenching rate is approximately expressed by the linear velocity of the copper roller because it is too difficult to measure a real quenching rate, i.e. cooling rate of the sample during quenching. The quenching rates used in the experiment were 5, 10, 15, 20, 25 and 30 m/s, respectively. The nominal compositions of the experimental alloys were La0.5Ce0.2Mg0.3Co0.4Ni2.6-xMnx (x=0, 0.1, 0.2, 0.3, 0.4), and for convenience, the alloys were represented with Mn content as Mn0, Mn1, Mn2, Mn3 and Mn4, respectively.
The cast ingot and quenched flakes were mechanically crushed and ground into the powder of less than 50 μm. The phase structures and compositions of the alloy powders were determined by XRD diffractometer (D/max/2400). The diffraction, with the experimental parameters of 160 mA, 40 kV and 10 (?)/min was performed with Cu Kα1 radiation filtered by graphite. The samples of the as-cast alloys were directly polished, and the flakes of the as-quenched alloys were enclosed in epoxy resin for polishing. The samples as- prepared were etched with a 60% HF solution. The morphologies of the as-cast and quenched alloys were examined by scanning electron microscope(SEM) (Philips QUANTA 400). The thin film samples of the as-quenched alloys were prepared for observing the grain morphology with the transmission electron microscope (TEM) (JEM-2100F, operated at 200 kV), and for determining the crystalline state of the samples with selected area electron diffraction(SAED).
Round electrode pellets of 15 mm in diameter were prepared by cold pressing a mixture of 0.2 g alloy powder and carbonyl nickel powder in a mass ratio of 1?4 under a pressure of 35 MPa for 5 min. After dried for 4 h, the electrode pellets were dipped in a 6 mol/L KOH solution for 24 h in order to wet fully the electrodes before the electrochemical measurement.
A tri-electrode open cell, consisting of a metal hydride electrode, a sintered NiOOH/Ni(OH)2 counter electrode and a Hg/HgO reference electrode, was used for testing the electrochemical characteristics of the experimental alloy electrodes. A 6 mol/L KOH solution was used as electrolyte. The voltage between the negative electrode and the reference electrode was defined as the discharge voltage. In every cycle, the alloy electrode was first charged with a constant current density, then after resting for 15 min, it was discharged at the same current density to -0.500 V cut-off voltage. The environment temperature of the measurement was kept at 30 ℃.
3 Results and discussion
3.1 Effect of rapid quenching on structural characteristics
The XRD patterns of the as-cast and quenched (30 m/s) alloys shown in Fig.1 indicate that all the alloys have multiphase structures, consisting of two major phases, (La, Mg)Ni3 and LaNi5, and a residual phase LaNi. The rapid quenching exerts an unconscious influence on the phase compositions of the alloys. The lattice parameters and phase abundances of LaNi5 and (La, Mg)Ni3 major phases in the as-cast and quenched Mn0 and Mn4 alloys are listed in Table 1, which were calculated from the XRD data by the software Jade 6.0. It can be derived from Table 1 that the rapid quenching leads to c axis increase and a axis and cell volume of LaNi5 and (La, Mg)Ni3 main phases slightly decrease and that it leads to an increase of LaNi5 phase and a decrease of (La, Mg)Ni3 phase. When the quenching rate increases from 0 (as-cast was defined as quenching rate of 0 m/s) to 30 m/s, LaNi5 phase increases from 27.16% to 31.01% for the Mn0 alloy, and from 38.28% to 40.72% for the Mn4 alloy. But (La, Mg)Ni3 phase decreases from 71.34% to 67.39% for the Mn0 alloy, and from 59.02% to 57.02% for the Mn4 alloy. In order to distinctly show the influence of quenching rate on the abundance of major phases in the alloys, the quenching rate dependence of the abundances of LaNi5 and (La,Mg)Ni3 major phases in the Mn0 and the Mn4 alloys is plotted in Fig.2. It can clearly be seen in Fig.2 that the rapid quenching leads to an increase of LaNi5 phase and a decrease of (La, Mg)Ni3 phase. For a fixed quenching rate, the amount of LaNi5 in the Mn0 alloys is less than that in the Mn4 alloy, and the amount of (La, M)Ni3 in the Mn0 alloys is more than that in the Mn4 alloy, suggesting that the substitution of Mn for Ni leads to an increase of LaNi5 phase and a decrease of (La, Mg)Ni3 phase.
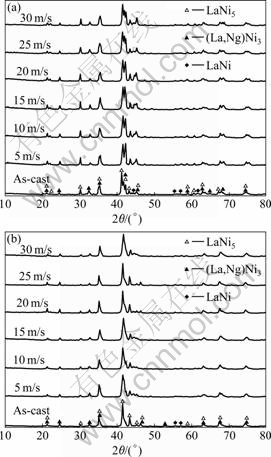
Fig.1 XRD profiles of as-cast and quenched alloys: (a) Mn0 alloy; (b) Mn4 alloy
Table 1 Lattice constants and abundances of LaNi5 and (La, Mg)Ni3 major phases

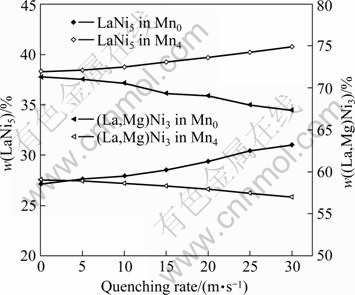
Fig.2 Evolution of abundances of major phases LaNi5 and (La,Mg)Ni3 with quenching rate
The SEM images of the as-cast and quenched (15 m/s) M0 and Mn4 alloys are shown in Fig.3. The result obtained by SEM with an energy dispersive spectrometry (EDS) indicates that all the experimental alloys are of multiphase structure, containing both (La,Mg)Ni3 and LaNi5 phases, which is in agreement with the results by XRD. Because the amount of LaNi phase is small and it attaches itself to (La, Mg)Ni3 phase in the process of growing, so, it is difficult to observe the morphology of LaNi phase. As shown in Fig.3, the as-cast alloys display coarse grains and poor composition homogeneity. The rapid quenching basically eliminates these structural defects of the as-cast alloys.
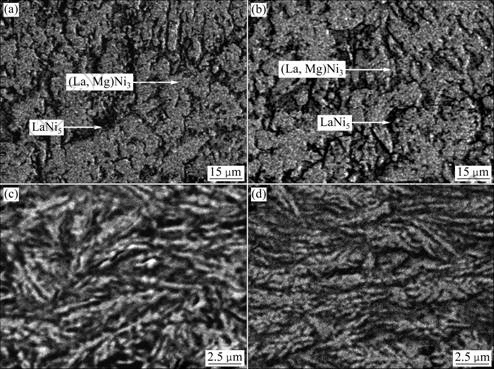
Fig.3 SEM images of as-cast and quenched (15 m/s) alloys: (a) As-cast Mn0 alloy; (b) As-cast Mn4 alloys; (c) As-quenched Mn0 aloy; (d) As-quenched Mn4 alloy
The morphologies and crystalline states of the as-quenched Mn4 alloy were examined by TEM, as shown in Fig.4. The figure exhibits that both the as-quenched alloys have nano-crystalline structures and the sizes of the grains are in the range of 20-30 nm.
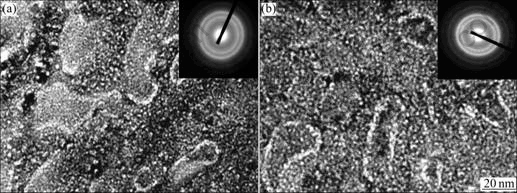
Fig.4 TEM morphologies and SAED patterns of as-quenched Mn2 alloys: (a) 20 m/s; (b) 30 m/s
3.2 Effect of rapid quenching on electrochemical characteristics
3.2.1 High rate discharge(HDR) ability
The high rate discharge(HRD) ability of the alloy
electrode, which was mainly determined by the kinetic property, was calculated according to following formula: HRD=C600, max/C100, max×100%, where C600,max and C100,max are the maximum discharge capacities of the electrode charged-discharged at the current densities of 600 and 100 mA/g, respectively. The quenching rate dependence of HRDs of the alloys is shown in Fig.5. It can be seen that the HRDs of the alloys first increase and then decrease with increasing quenching rate. The HRDs of the alloys have the maximum values with the variety of quenching rate, which is known as the optimal quenching rate, and it changes with the variety of Mn content.
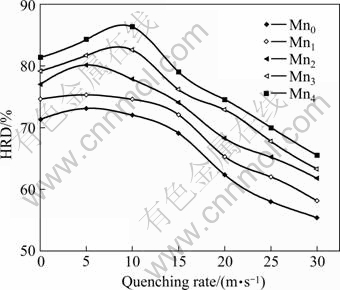
Fig.5 Evolution of high rate discharge(HRD) capabilities of alloys with quenching rate
The HRD is a dynamic problem of hydrogen absorbing/desorbing of the alloy electrode, which is influenced mainly by the electrochemical reaction kinetics on the alloy powder surface and the diffusion rate of hydrogen in the bulk of the alloy[18]. The rapid quenching leads to an increase of LaNi5 phase, which significantly enhances the electrocatalytic activity of alloy electrode. The grain refinement produced by rapid quenching enhances the diffusion capability of hydrogen in the alloy[19], but the lattice distortion and internal stress caused by rapid quenching lower the diffusion capability of hydrogen[20]. It seems to be self-evident that the above contrary effects consequentially result in an optimum quenching rate for the HRDs of the alloys.
3.2.2. Discharge capacity
The evolution of the maximum discharge capacities of the alloys with quenching rate is shown in Fig.6, for a charge-discharge current density of 100 mA/g. Fig.6 shows that the maximum discharge capacity of the alloys first increases and then decreases with the increase of the quenching rate. The discharge capacity of the alloys obtains the greatest values at a special quenching rate named the optimal quenching rate, and the optimal quenching rate is changeable with the variety of Mn content. The discharge capacity increases from 364.06 mA?h/g (0 m/s) to 371.34 mA?h/g (5 m/s), after that it drops to 334.65 mA?h/g (30 m/s) for the Mn0 alloy, and mounts up from 382.01 mA?h/g (0 m/s) to 385.38 mA?h/g (5 m/s), and then declines to 351.32 mA?h/g (30 m/s) for the Mn2 alloys. The discharge capacity of the alloys has the maximum value with the variety of quenching rate, which is relevant to the change of alloy structure caused by rapid quenching. The decrease of a cell volume caused by rapid quenching is unfavourable for the discharge capacity of the alloy, but the decrease of grain size of the alloy and the improvement of composition homogeneity of the alloy produced by rapid quenching are helpful for the discharge capacity of the alloy. The amount of LaNi5 in the alloy increases with increasing quenching rate, which is disadvantageous to the discharge capacity of the alloy due to the fact that the discharge capacity of LaNi5 phase is less than that of (La, Mg)Ni3 phase[21]. However, it is noteworthy that LaNi5 phase works not only as a hydrogen reservoir but also as a catalyst to activate (La,Mg)Ni3 phase to absorb/desorb reversibly hydrogen in alkaline electrolyte[22]. It is the above contrary effects that result in an optimum quenching rate for the discharge capacities of the alloys.

Fig.6 Evolution of discharge capacities of alloys with quenching rate
3.2.3 Activation capability
The quenching rate dependence of the activation number of the electrode alloys is shown in Fig.7. The figure indicates that all the as-cast and quenched alloys display excellent activation performances and can attain their maximum discharge capacities after 2 to 5 charging-discharging cycles. The rapid quenching slightly impairs the activation capabilities of the alloys. Basically, the activation capability of hydrogen storage alloy is directly relevant to the change of internal energy of the hydride system before and after absorbing hydrogen. The larger the additive internal energy, involving the surface energy which is originated from the oxidation film formed on the surface of the electrode alloy and the strain energy which is produced by hydrogen atom entering the interstitials of the tetrahedron or octahedron of the alloy lattice, the poorer the activation performance of the alloy[23]. The reason why rapid quenching impairs the activation performances of the alloys is attributed to the reduction of cell volumes of the alloys caused by rapid quenching, increasing the ratios of expansion/contraction of the alloys in the process of hydrogen absorption/desorption, which means increasing the strain energy. Although the increase of LaNi5 phase caused by rapid quenching is helpful for the activation performance, the negative impact produced by rapid quenching is obviously more predominant.
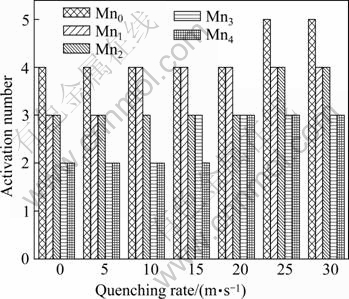
Fig.7 Relationship between quenching rate and activation number
3.2.4 Cycle stability
The cycle life is defined as the cycle number after which the discharge capacity of the alloy at a current density of 600 mA/g is reduced to 60% of the maximum capacity. Fig.8 shows the evolution of discharge capacities of the as-cast and quenched Mn2 alloy with cycle number. A tendency can be seen in Fig.8 that the decay rate of discharge capacity of the Mn2 alloy decreases with increasing quenching rate, suggesting that the rapid quenching enhances the cycle stability of the alloy. The capacity retaining rate (S100), which is introduced to evaluate accurately the cycle stability of the alloy, is defined as S100=C100/Cmax×100%, where Cmax is the maximum discharge capacity, and C100 is the discharge capacity of the 100th cycle at a current density of 600 mA/g, respectively. The capacity retaining rates (S100) of the alloys as a function of quenching rate are shown in Fig.9. It can be seen in Fig.9 that rapid quenching leads to a significant increase of capacity retaining rate (S100) of the alloy. When the quenching rate rises from 0 to 30 m/s, the capacity retaining rate (S100) increases from 67.71% to 83.77% for the Mn0 alloy, and from 62.04% to 78.51% for the Mn4 alloy. It can also be derived in Fig.9 that for a fixed quenching rate, the capacity retaining rates (S100) of the alloys decline with the increase of Mn content, meaning that the substitution of Mn for Ni clearly impairs the cycle stabilities of the alloys.
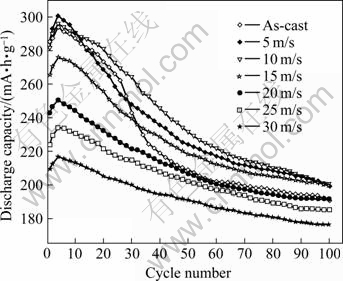
Fig.8 Evolution of discharge capacities of Mn2 alloy with cycle number
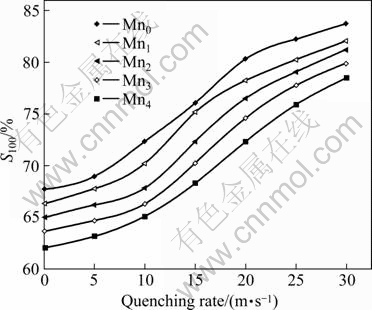
Fig.9 Evolution of capacity retaining rates (S100) of alloys with quenching rate
The electrode failure is characterized by the decay of discharge capacity and the drop of discharge voltage. Refs.[24-25] revealed that the fundamental reasons for the capacity decay of electrode alloy were the pulveriza- tion and oxidation of alloy electrode during charging-discharging cycle. The lattice stress and the expansion of the cell volume, which are inevitable when hydrogen atoms enter into the interstitials of lattice, are the real driving force that leads to the pulverization of the alloy. The positive impact of rapid quenching on the cycle stability of the alloy is primarily ascribed to the significant refinement of grains caused by rapid quenching. The anti-pulverization capability of the alloy basically depends on its strength. The smaller the grain size, the higher the strength of the alloy. Therefore, it is understandable that the cycle stability of the alloy increases with increasing quenching rate. The substitution of Mn for Ni significantly impairs the anti-corrosion capability of the alloy electrode[26], which is responsible for the slight decrease of the cycle stability of the alloy caused by Mn substitution.
4 Conclusions
1) The rapid quenching does not change the phase compositions of La0.5Ce0.2Mg0.3Co0.4Ni2.6-xMnx (x=0, 0.1, 0.2, 0.3, 0.4) electrode alloys, but it leads to an increase of LaNi5 phase and a decrease of (La, Mg)Ni3 phase in the alloys. The rapid quenching basically eliminates the structural defects of the as-cast alloy, involving coarse grains and poor composition homogeneity.
2) The rapid quenching has significant influences on the electrochemical performances of the alloys. The discharge capacities and high rate discharge(HRD) abilities of the alloys have the maximum values for a special quenching rate, which is changeable with the variety of Mn content. The rapid quenching significantly improves the cycle stabilities of the alloys, but it slightly impairs the activation performances of the alloys.
References
[1] WILLEMS J J G, BUSCHOW K H J. A nickel metal hydride battery for electric vehicles [J]. J Less-Common Met, 1987, 129: 13-30.
[2] OVSHINSKY S R, FETCENKO M A, ROSS J. From permanent magnets to rechargeable hydride electrodes [J]. Science, 1993, 260: 176-181.
[3] TSUKAHARA M, KAMIYA T, TAKAHASHI K, KAWABATA A, SAKURAI S, SHI J, TAKESHITA H T, KURIYAMA N, SAKAI T. Hydrogen storage and electrode properties of V-based solid solution type alloys prepared by a thermic process [J]. J Electrochem Soc, 2000, 147: 2941-2944.
[4] SUN D L, ENOKI H, GINGL F, AKIBA E. New approach for synthesizing Mg-based alloys [J]. J Alloys Comp, 1999, 285: 279-283.
[5] KADIR K, SAKAI T, UEHARA I. Synthesis and structure determination of a new series of hydrogen storage alloys: RMg2Ni9 (R=La, Ce, Pr, Nd, Sm and Gd) built from MgNi2 Laves-type layers alternating with AB5 layers [J]. J Alloys Comp, 1997, 257: 115-121.
[6] KADIR K, KURIYAMA N, SAKAI T, UEHARA I, ERIKSSON L. Structural investigation and hydrogen capacity of CaMg2Ni9: A new phase in the AB2C9 system isostructural with LaMg2Ni9 [J]. J Alloys Compd, 1999, 284: 145-154.
[7] KADIR K, SAKAI T, UEHARA I. Structural investigation and hydrogen capacity of YMg2Ni9 and (Y0.5Ca0.5)(MgCa)Ni9: New phases in the AB2C9 system isostructural with LaMg2Ni9 [J]. J Alloys Comp, 1999, 287: 264-270.
[8] KOHNO T, YOSHIDA H, KAWASHIMA F, INABA T, SAKAI I, YAMAMOTO M, KANDA M. Hydrogen storage properties of new ternary system alloys: La2MgNi9, La5Mg2Ni23, La3MgNi14 [J]. J Alloys Comp, 2000, 311: L5-L7.
[9] LIAO B, LEI Y Q, LU G L, CHEN L X, PAN H G, WANG Q D. The electrochemical properties of LaxMg3-xNi9 (x=1.0–2.0) hydrogen storage alloys [J]. J Alloys Comp, 2003, 356/357: 746-749.
[10] WANG D H, LUO Y C, YAN R X, ZHANG F L, KANG L. Phase structure and electrochemical properties of La0.67Mg0.33Ni3.0-xCox (x=0, 0.25, 0.5, 0.75) hydrogen storage alloys [J]. J Alloys Comp, 2006, 413:193-197.
[11] TANG R, LIU Y N, ZHU C, ZHU J W, YU G. Effect of Al substitution for Co on the hydrogen storage characteristics of Ml0.8Mg0.2Ni3.2Co0.6-xAlx (x=0–0.6) alloys [J]. Intermetallics, 2006, 14: 361-366.
[12] ZHANG X B, SUN D Z, YIN W Y, CHAI Y J, ZHAO M S. Crystallographic and electrochemical characteristics of La0.7Mg0.3Ni3.5-x(Al0.5Mo0.5)x (x=0–0.8) hydrogen storage alloys [J]. J Power Sources, 2006, 154: 290-297.
[13] PAN H G, YUE Y J, GAO M X, WU X F, CHEN N, LEI Y Q, WANG Q D. The effect of substitution of Zr for La on the electrochemical properties of La0.7-xZrxMg0.3Ni2.45Mn0.1Co0.75Al0.2 hydrogen storage electrode alloys [J]. J Alloys Comp, 2005, 397: 269-275.
[14] PAN H G, LIU Y F, GAO M X, WANG Q D. Electrochemical properties of the La0.7Mg0.3Ni2.65–xMn0.1Co0.75Alx (x=0-0.5) hydrogen storage alloy electrodes [J]. J Electrochem Soc, 2005, 152(2): A326-A332.
[15] ZHANG Y H, CHEN M Y, WANG X L, WANG G Q, DONG X P, QI Y. Microstructure and electrochemical characteristics of Mm(Ni,Co,Mn,Al)5Bx (x=0–0.4) hydrogen storage alloys prepared by cast and rapid quenching [J]. Electrochimica Acta, 2004, 49: 1161-1168.
[16] ZHANG Y H, WANG G Q, DONG X P, GUO S H, WANG X L. Effect of rapid quenching on the microstructures and electrochemical performances of Co-free AB5-type hydrogen storage alloys [J]. Int J Hydrogen Energy, 2005, 30: 091-1098.
[17] ZHANG Y H, WANG G Q, DONG X P, GUO S H, WANG X L. Effects of rapid quenching on the electrochemical performances and microstructures of the Mm(NiMnSiAl)4.3Co0.6-xFex (x=0–0.6) electrode alloys [J]. J Power Sources, 2004, 137: 309-316.
[18] IWAKURA C, MATSUOKA M, ASAI K, KOHNO T. Surface modification of metal hydride negative electrodes and their charge/discharge performance [J]. J Power Source, 1992, 38: 335-343.
[19] LI P, WANG X L, ZHANG Y H, LI R, WU J M, QU X H. Research on electrochemical characteristics and microstructure of Mm(NiMnAl)4.9Co0.2 rapidly quenched alloy [J]. J Alloys Comp, 2003, 353: 278-282.
[20] ZHANG Y H, WANG G Q, DONG X P, GUO S H, WANG X L. Effects of substituting Co with Fe on the microstructures and electrochemical performances of the as-cast and quenched AB5-type hydrogen storage alloys [J]. Functional Materials, 2006, 37(2): 250-254. (in Chinese)
[21] LIU Y F, PAN H G, GAO M X, ZHU Y F, LEI Y Q. Hydrogen storage and electrochemical properties of the La0.7Mg0.3Ni3.825-xCo0.675Mnx hydrogen storage electrode alloys [J]. J Alloys Comp, 2004, 365: 246-252.
[22] PAN H G, LIU Y F, GAO M X, LEI Y Q, WANG Q D. Study of the structural and electrochemical properties of La0.7Mg0.3(Ni0.85Co0.15)x (x=2.5-5.0) hydrogen storage alloys [J]. J Electrochem Soc, 2003, 150: A565-A570.
[23] WU M S, WU H R, WANG Y Y, WAN C C. Surface treatment for hydrogen storage alloy of nickel/metal hydride battery [J]. J Alloys and Comp, 2000, 302: 248-257.
[24] LIU Y F, PAN H G, GAO M X, LEI Y Q, WANG Q D. XRD study on the electrochemical hydriding/dehydriding behavior of the La-Mg-Ni-Co-type hydrogen storage alloys [J]. J Alloys Comp, 2005, 403: 296-304.
[25] CHARTOUNI D, MELI F, Z?TTEL A, GROSS K, SCHLAPBACH L. The influence of cobalt on the electrochemical cycling stability of LaNi5-based hydride forming alloys [J]. J Alloys Comp, 1996, 241: 160-166.
[26] MELI F, Z?TTEL A, SCHLABACH L. Electrochemical and surface properties of low cost, cobalt-free LaNi5-type hydrogen storage alloys [J]. J Alloys Comp, 1993, 202: 81-88.
Foundation item: Project(2006AA05Z132) supported by the Hi-tech Research and Development Program of China; Project(50701011) supported by the National Natural Science Foundation of China; Project(200711020703) supported by the Natural Science Foundation of Inner Mongolia, China; Project(NJzy08071) supported by Higher Education Science Research Project of Inner Mongolia, China
Corresponding author: ZHANG Yang-huan; Tel: +86-10-62187570; E-mail: zyh59@yahoo.com.cn
DOI: 10.1016/S1003-6326(08)60279-4
(Edited by YUAN Sai-qian)