J. Cent. South Univ. Technol. (2009) 16: 0380-0384
DOI: 10.1007/s11771-009-0064-9
Synthesis and optophysical properties of blue-emitting iridium (Ⅲ) complex bearing oxadiazole-based picolinic acid derivative
WEN Zhong-lin(文忠林)1, HU Zheng-yong(胡峥勇)1, LIU Yu(刘 煜)1, XIAO Fang-liang(肖方亮)1,
MA Xiao-yun(马小云)1, ZHU Mei-xiang(朱美香)1, ZHU Wei-guo(朱卫国)1, 2
(1. College of Chemistry, Xiangtan University, Xiangtan 411105, China;
2. Key Laboratory of Environment-Friendly Chemistry and Application, Ministry of Education,
Xiangtan University, Xiangtan 411105, China)
Abstract: An iridium (Ⅲ) bis[(4,6-difluorophenyl)pyridinato-N, C2][6-(6′-(4″-(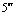 -phenyl-
-phenyl-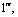
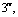
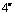 -oxadiazole-
-oxadiazole-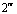 -yl) phenoxy) hexyloxy picolinate) was synthesized and characterized by 1H NMR and elementary analysis in order to study the effect of ancillary ligand of the oxadiazole-based picolinic acid derivative on optophysical properties of its iridium complex, and further to obtain an iridium complex with highly-efficient blue emission. The thermal stability, UV absorption and photoluminescent properties of this iridium complex were investigated. Compared with iridium (Ⅲ) bis[(4,6-difluorophenyl)pyridinato-N, C2](picolinate) reported as a highly-efficient blue electroluminescent material, this iridium complex bearing an oxadiazole-based picolinic acid derivative presents higher thermal stability, more intense UV absorption at 291 nm and similar photoluminescent spectrum peaked at 469 nm. This indicates that tuning ancillary ligand of picolinic acid with an oxadiazole unit can improve the optophysical properties of its iridium complex.
-yl) phenoxy) hexyloxy picolinate) was synthesized and characterized by 1H NMR and elementary analysis in order to study the effect of ancillary ligand of the oxadiazole-based picolinic acid derivative on optophysical properties of its iridium complex, and further to obtain an iridium complex with highly-efficient blue emission. The thermal stability, UV absorption and photoluminescent properties of this iridium complex were investigated. Compared with iridium (Ⅲ) bis[(4,6-difluorophenyl)pyridinato-N, C2](picolinate) reported as a highly-efficient blue electroluminescent material, this iridium complex bearing an oxadiazole-based picolinic acid derivative presents higher thermal stability, more intense UV absorption at 291 nm and similar photoluminescent spectrum peaked at 469 nm. This indicates that tuning ancillary ligand of picolinic acid with an oxadiazole unit can improve the optophysical properties of its iridium complex.
Key words: iridium complex; 1, 3, 4-oxadiazole; synthesis; optophysical properties
1 Introduction
Cyclometalated iridium complexes have been extensively studied because of their unique internal quantum efficiency and colorful emission across the visible spectrum easily tuned by structure changes, as well as potential application in phosphorescent organic and polymeric light-emitting devices [1-3]. High- efficiency green and red emissions from cyclometalated iridium complexes have been presented, but the blue-emitting cyclometalated iridium complexes have not displayed satisfactory emission efficiency in these devices [4-9]. As organic and polymeric light-emitting devices have a significant application in full-color displays and solid lights, and blue emission is the best one among the three colors, the blue-emitting iridium complexes still need to be developed.
To efficiently obtain blue-emitting iridium complexes, it is critical to make clear the relationship between molecular structure and luminescent property. In the previous reports, SAJOTO et al [10] and HOLMES et al [11] showed blue and near-UV phosphorescence from the iridium complexes with pyrazolyl and N-heterocyclic carbene cyclometalated ligands. LO et al [12] subsequently demonstrated deep-blue phosphorescent emission from the iridium complexes containing phenyltriazole cyclometalated ligands. TAKIZAWA et al [13] described a series of blue-emitting iridium complexes with 2-phenylimidazo[1, 2-a]pyridine cyclometalated ligand. LI et al [14] developed a class of non-conjugate dendritic iridium complexes containing polybenzyloxy pyridine cyclometalated ligands to emit blue light. Most of these blue-emitting iridium complexes are achieved by modification of cyclometalated ligands rather than ancillary ligands.
KWONG et al [15] reported a high-efficiency iridium complex bearing a picolinic acid derivative modified by carbazoyl dendrimeric group with hole- transporting properties. In this work, a blue-emitting iridium (Ⅲ) complex was designed, in which a phenyl oxadiazole moiety with electron-transporting properties was introduced into the ancillary ligand of picolic acid. The resulting complex is iridium (Ⅲ) bis [(4, 6- difluorophenyl)pyridinato-N, C2] [6-(6′- (4″-(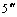 -phenyl-
-phenyl-  phenoxy) hexyloxy picolinate), (dfppy)2Ir(OXD-OHO-Pic). The synthetic route of this complex is shown in Scheme 1. Incorporating an oxadiazole unit into picolinic acid to obtain a new ancillary ligand, its iridium complex was considered due to the reason that oxadiazole derivatives are good candidates for electron injection and transportation in light-emitting devices [16]. Introducing an oxadiazole unit into cyclometalated ligands has presented good results in enhancing the optoelectronic properties of their iridium complexes [17-18]. As oxadiazole moiety has a potential to improve the electron-transporting properties of its iridium complex and bis[(4, 6-difluorophenyl) pyridinato-N, C2] (picolinate), (dfppy)2Ir(Pic) is one of the phosphorescent materials with highly-efficient blue emission, the resulting (dfppy)2Ir(OXD-OHO-Pic) complex is expected to become a promising blue- emitting electroluminescence material.
phenoxy) hexyloxy picolinate), (dfppy)2Ir(OXD-OHO-Pic). The synthetic route of this complex is shown in Scheme 1. Incorporating an oxadiazole unit into picolinic acid to obtain a new ancillary ligand, its iridium complex was considered due to the reason that oxadiazole derivatives are good candidates for electron injection and transportation in light-emitting devices [16]. Introducing an oxadiazole unit into cyclometalated ligands has presented good results in enhancing the optoelectronic properties of their iridium complexes [17-18]. As oxadiazole moiety has a potential to improve the electron-transporting properties of its iridium complex and bis[(4, 6-difluorophenyl) pyridinato-N, C2] (picolinate), (dfppy)2Ir(Pic) is one of the phosphorescent materials with highly-efficient blue emission, the resulting (dfppy)2Ir(OXD-OHO-Pic) complex is expected to become a promising blue- emitting electroluminescence material.
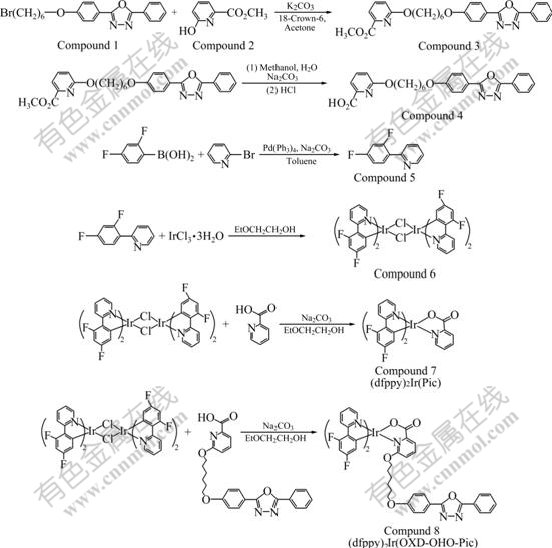
Scheme 1 Synthetic route of (dfppy)2Ir(OXD-OHO-Pic)
2 Experimental
2.1 Instruments and reagents
All 1H NMR spectra were acquired on a Bruker Dex-400 NMR instrument using CDCl3 as solvent. Elemental analysis was performed with a Perkin-Elmer 240 instrument. UV-Vis absorption spectra were recorded with an HP-8453 UV visible system. The films of the iridium complexes were formed on the quartz under pressure. Photoluminescence (PL) spectrum was recorded on a fluorescence spectrophotometer (HITACHI-850) under excitation of 325 nm line. Thermo-gravimetric analysis (TGA) was carried out with a NETZSCH STA449 from 25 to 700 ℃ at a heating rate of 20 ℃/min under nitrogen.
All chemicals used were of analytical grade. Solvents were puri?ed with conventional methods. 2-(4- (6-bromohexyloxy)phenyl)-5-phenyl-1, 3, 4-oxadiazole (Compound 1) was synthesized according to Ref.[19].
2.2 Synthesis of methyl 6-(6′-(4″-(5′″-phenyl-1′″, 3′″, 4′″-oxadiazole-2′″-yl) phenoxy) hexyloxy picolinate (Compound 3)
A mixture of 2-(4-(6-bromohexyloxy)phenyl)-5- phenyl-1, 3, 4-oxadiazole (1.31 g, 3.27 mmol), methyl-6- hydroxypicolinate (0.5 g, 3.27 mmol) and potassium carbonate (2.26 g, 16.35 mmol) in DMF (50 mL) was stirred at 80 ℃ for 20 h. After being cooled to ambient temperature, the mixture was treated with 100 mL of water. The aqueous layer was separated and extracted with 50 mL dichlomethylene three times. The resulting organic layer was dried over MgSO4 and concentrated in vacuo. The residue was purified by flash chromatography with an eluent CH2Cl2 to provide 0.98 g Compound 3 as white solid powder in a 63.3% yield. 1H NMR (400 MHz, CDCl3, chemical shift δ): 1.56-1.57 (m, 4H), 1.84-1.86 (m, 4H), 3.96 (s, 3H), 4.06 (t, 2H), 4.41 (t, 2H), 6.90 (t, 1H), 7.01 (d, 2H, J = 8.72 Hz), 7.53-7.54 (m, 3H), 7.67 (t, 2H), 8.06-8.14 (m, 4H).
2.3 Synthesis of 6-(6′-(4″-(5′″-phenyl-1′″, 3′″, 4′″- oxadiazole-2′″-yl) phenoxy) hexyloxy) picolinic acid (OXD-OHO-Pic) (Compound 4)
It was synthesized with a 86.3% yield as white powder according to Ref.[20]. 1H NMR (400 MHz, CDCl3, chemical shift δ): 8.06-8.14 (m, 4H), 7.67 (t, 2H), 7.53-7.54 (m, 3H), 7.01 (d, 2H, J=8.72 Hz), 6.90 (dd, 1H, J1=3.47 Hz, J2=3.51 Hz), 4.41 (dd, 2H, J1=6.53 Hz, J2=6.36 Hz), 4.06 (dd, 2H, J1=6.38 Hz,J2=6.42 Hz), 1.84-1.86 (m, 4H), 1.56-1.57 (m, 4H).
2.4 Synthesis of [(dfppy)2Ir(μ-Cl)]2
It was synthesized according to Ref.[21]. 1H NMR (400 MHz, CDCl3, chemical shift δ): 7.25 (m, 1H), 7.75 (m, 2H), 8.01 (m, 1H), 8.70 (d, 1H, J = 4.34 Hz).
2.5 Synthesis of (dfppy)2Ir(OXD-OHO-Pic)
A mixture of OXD-OHO-Pic (0.16 g, 0.35 mmol), the dimmer [(dfppy)2Ir(μ-Cl)]2 (0.17 g, 0.14 mmol), sodium carbonate(0.15 g, 1.41 mmol) and 2-ethoxy- ethanol (20 mL) was refluxed under nitrogen atmosphere for 10 h and evaporated. The residue was dissolved in ethylacetate and the resulting organic solution was washed with water and dried over MgSO4, then concentrated. The residue was purified by flash chromatography with an eluent of ethyl acetate to give a yellow powder (0.11 g) with a yield of 82.3%. 1H NMR (400 MHz, CDCl3, chemical shift δ): 1.30-1.36 (m, 4H), 1.92-1.94 (m, 4H), 3.64 (t, 2H), 3.95 (t, 2H), 5.35 (d, 1H, J=3.16 Hz), 5.67 (d, 1H, J=3.40 Hz), 6.32 (m, 1H), 6.88 (m, 1H), 6.94 (m, 3H), 7.05 (m, 1H), 7.08 (m, 1H), 7.46 (m, 4H), 7.58 (m, 1H), 7.68 (m, 1H), 7.86 (m, 1H), 7.95 (m, 1H), 8.01 (m, 2H), 8.07 (m, 2H), 8.17 (m, 2H), 8.61 (m, 1H). IrC48H36F4N5O5 Calcd. (mass fraction, %): C 60.00, H 3.75, N 7.29; Found (mass fraction, %): C 59.96, H 3.73, N 7.26.
3 Results and discussion
3.1 Synthesis of iridium complex
The (dfppy)2Ir(OXD-OHO-Pic) complex was synthesized by chlorine-bridged and debridged reactions according to Refs.[20-22]. Elemental analysis and 1H NMR of the (dfppy)2Ir(OXD-OHO-Pic) complex are consistent with the expected molecular structure. A yield of 82.3% in the debridged reaction between the [(dfppy)2Ir(μ-Cl)]2 dimer and the OXD-OHO-Pic ancillary ligand is obtained, which is 20% higher than that between the [(dfppy)2Ir(μ-Cl)]2 dimer and picolinic acid. This indicates that ancillary ligand has an influence on the debridged reaction. The introduction of non-conjugated alkoxy chain and oxadiazole unit in picolinic acid can effectively increase the debridged effect of the ancillary ligand and further increase the yield of iridium complex because the OXD-OHO-Pic ancillary ligand has a better solubility and an increased acidity compared to picolic acid.
3.2 Thermal properties
The thermal properties of the (dfppy)2Ir(OXD- OHO-Pic) complex were determined by thermo- gravimetric analysis (TGA). The TGA curves of the (dfppy)2Ir(OXD-OHO-Pic) and (dfppy)2Ir(Pic) com- plexes are shown in Fig.1. The (dfppy)2Ir(OXD-OHO- Pic) complex displays high thermal stability like the (dfppy)2Ir(Pic) complex. No mass loss is observed up to 300 ℃ for this complex. The (dfppy)2Ir(OXD-OHO- Pic) complex is decomposed and gives a mass loss of 50.6% at 439 ℃. The mass loss is attributed to the removal of the OXD-OHO-Pic ancillary ligand from the iridium complex. This indicates that tuning piconilic acid derivative by a non-conjugated alkoxy chain and an oxadiazole moiety has a minor effect on thermal stability of its iridium complex. As a result, this kind of iridium
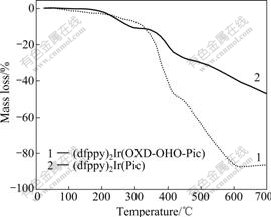
Fig.1 TGA curves of (dfppy)2Ir(OXD-OHO-Pic) and (dfppy)2Ir(Pic) complexes recorded at heating rate 20 ℃/min in dynamic N2 atmosphere
complex containing an oxadiazole-based piconilic acid derivative exhibits good thermal stability.
3.3 UV-Vis absorption spectra
Fig.2 shows UV absorption spectra of the (dfppy)2Ir(OXD-OHO-Pic) and (dfppy)2Ir(Pic) complexes in dichlomethene. The (dfppy)2Ir(Pic) complex exhibits an intense absorption peak at 300 nm and a weak absorption band at 380 nm. The intense absorption at 300 nm is assigned to the spin allowed π-π* electron transition. The weak absorption band at 380 nm corresponds to the metal-ligand charge transfer (MLCT) transition. In contrast, (dfppy)2Ir(OXD-OHO-Pic) exhibits a minor blue-shifted and enhanced absorption peak at 291 nm although there is a similar absorption band at 380 nm for these two iridium complexes. This indicates that the ancillary ligand modified by oxadiazole group can improve UV properties of its iridium complex. The enhanced UV absorption of (dfppy)2Ir(OXD-OHO- Pic) is related to incorporation of oxadiazole group in the picolinic acid, which is useful for this iridium complex to emit light with high-efficiency in the phsophorescent doped devices.
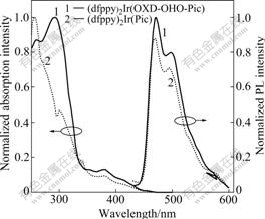
Fig.2 UV absorption spectra and photoluminescence (PL) spectra of (dfppy)2Ir(OXD-OHO-Pic) and (dfppy)2Ir(Pic) complexes
3.4 Photoluminescence spectra
In order to compare with the PL characteristics between (dfppy)2Ir(OXD-OHO-Pic) and (dfppy)2Ir(Pic), the PL spectra of these two iridium complexes in dichloromethane are also shown in Fig.2. Intense blue emission and similar PL profiles are observed. The maximum emission peak at 469 nm and a shoulder at 497 nm are displayed in both PL profiles. In our previous research, we found that picolinic acid directly connected with phenyl oxadiazole made its iridium complex have red-shifted PL emission peak [20]. This implies that the non-conjugated hexyloxy chain between the conjugated phenyl oxadiazole moiety and picolinic acid can effectively break the conjugated effect of these two aryl groups in the picolinic acid derivative, further avoid red-shifted emission of its iridium complex. The introduction of phenyl oxadiazole moiety into the common picolinic acid bridged with a non-conjugated hexyloxy chain is an effective way to obtain new blue-emitting materials. As oxadiazole group had excellent electron-transporting property, it improved optoelectronic properties of its iridium complex while it was introduced into cyclometalated ligand [17-18]. In addition, the blue-emitting (dfppy)2Ir(Pic) complex displayed excellent electroluminescent properties in organic and polymer light-emitting devices and the (dfppy)2Ir(OXD-OHO-Pic) complex displayed similar and intense blue PL profile like (dfppy)2Ir(Pic). Therefore, (dfppy)2Ir(OXD-OHO-Pic) should exhibit improved optoelectronic properties and become a promising blue-emitting electroluminescent material. In order to confirm this suggestion, the electro-luminescent properties of these iridium complexes in doped devices will be studied.
4 Conclusions
(1) An ancillary ligand OXD-OHO-Pic and its (dfppy)2Ir(OXD-OHO-Pic) complex are achieved. Intense UV absorption and blue emission in dichlomethane, as well as high thermal stability in solid state are observed for this iridium complex.
(2) Compared with the (dfppy)2Ir(Pic) complex, the (dfppy)2Ir(OXD-OHO-Pic) complex displays good optophysical properties. Therefore, modifying the traditional ancillary ligands with the oxadiazole unit is a simple way to obtain the blue-emitting electro- luminescent materials.
References
[1] Baldo M A, Lamansky S, Burrows P E, Thompson M E, Forrest S R. Very high-efficiency green organic light-emitting devices based on electrophosphorescence [J]. Appl Phys Lett, 1999, 75(1): 4-6.
[2] Adachi C, Baldo M A, Forrest S R, Lamansky S, Thompson M E, Kwong R C. High-efficiency red electro- phosphorescence devices [J]. Appl Phys Lett, 2001, 78(11): 1622- 1624.
[3] Ikai M, Tokito S, Sakamoto Y T. Suzuki T, Taga Y. Highly efficient phosphorescence from organic light-emitting devices with an exciton-block layer [J]. Appl Phys Lett, 2001, 79(2): 156- 158.
[4] Laskar I R, Chen T M. Tuning of wavelength: Synthesis and photophysical studies of iridium complexes and their applications in organic light emitting devices [J]. Chem Mater, 2004, 16(1): 111- 117.
[5] Tsuzuki T, Shirasawa N, Suzuki T, Tokito S. Color tunable organic light emitting diodes using pentafluoro-ogenyl substituted iridium complexes [J]. Adv Mater, 2003, 15(17): 1455-1458.
[6] Tamayo A B, Alleyne B D, Djurovich P I, Lamansky S, Tsyba I, Ho N N, Bau R, Thompson M E. Synthesis and characterization of facial and meridional tris-cyclometalated iridium(Ⅲ) complexes [J]. J Am Chem Soc, 2003, 125(24): 7377- 7387.
[7] Holmes R J, Andrade W D, Forrest S R, Ren X, Li J, Thompson M E. Efficient, deep-blue organic electrophos phorescence by guest charge trapping [J]. Appl Phys Lett, 2003, 83(18): 3818-3820.
[8] Tokito S, Iijima T, Suzuri Y, Tsuzuki T, Sato F. Confinement of triplet energy on phosphorescent molecules for highly-efficient organic blue-light-emitting devices [J]. Appl Phys Lett, 2003, 83(3): 569-571.
[9] Dedeian K, Shi J, Shepherd N, Forsythe E, Morton D C. Photophysical and electrochemical properties of heteroleptic tris-cyclometalated iridium (Ⅲ) complexes [J]. Inorg Chem, 2005, 44(13): 4445-4447.
[10] Sajoto T, Djurovich P I, Tamayo A, Yousufuddin M, Bau R, Thompson M E. Blue and near-UV phosphorescence from iridium complexes with cyclometalated pyrazolyl or N-heterocyclic carbene ligands [J]. Inorg Chem, 2005, 44(22): 7992- 8003.
[11] Holmes R J, Forrest S R, Sajoto T, Tamayo A, Djurovich P I, Thompson M E, Brooks J, Tung Y J, D’Andrade B W, Weaver M S, Kwong R C, Brown J J. Saturated deep blue organic electro-phosphorescence using a fluorine-free emitter [J]. Appl Phys Lett, 2005, 87(24): 243507/1- 243507/3.
[12] Lo S C, Shipley C P, Bera R N, Harding R E, Cowley A R, Burn P L, Samuel D W. Blue phosphorescence from iridium complexes at room temperature [J]. Chem Mater, 2006, 18(21): 5119-5129.
[13] Takizawa S Y, Nishida J, Tsuzuki T, Tokito S, Yamashita Y. Phosphorescent iridium complexes based on 2-phenylimidazo [1, 2-a] pyridine ligands: Tuning of emission color toward the blue region and application to polymer light-emitting devices [J]. Inorg Chem, 2007, 46(10): 4308-4319.
[14] Li X H, Chen Z, Zhao Q, Shen L, Li F Y, Yi T, Cao Y, Huang C. Nonconjugated dendritic iridium (Ⅲ) complexes with tunable pyridine-based ligands: Synthesis, photophysical, electro- chemical, and electroluminescent properties [J]. Inorg Chem, 2007, 46(14): 5518-5527.
[15] Kwong T, Kim M K, Kwong J, Shin D, Park S J, Lee C, Kim J, Hong J I. High efficiency light-harvesting system based on a phosphorescent acceptor coupled with dendrimer donors via singlet-singlet and triplet-triplet energy transfer [J]. Chem Mater, 2007, 19(15): 3673-3680.
[16] Hughes G, Bryce M B. Electron-transporting materials for organic electroluminescent and electrophosphorescent devices [J]. J Mater Chem, 2005, 15: 94-107.
[17] Xu Z W, Li Y, Ma X M, Gao X D, Tian H. Synthesis and properties of iridium complexes based 1, 3, 4-oxadiazoles derivatives [J]. Tetrahedron, 2008, 64(8): 1860-1867.
[18] Chen L Q, Yang C L, Qin J G, Gao J, You H, Ma D G. Synthesis, structure, electrochemistry, photophysics and electro- luminescence of 1, 3, 4-oxadiazole-based ortho-metalated iridium(Ⅲ) complexes [J]. J Organometal Chem, 2006, 691(16): 3519-3530.
[19] Jin J, Kim J Y, Park S H, Kim J, Lee S, Lee K, Suh H. Synthesis and properties of electroluminescent polyfluorene-based conjugated polymers containing oxadiazole and carbazole units as pendants for LEDs [J]. Polymer, 2005, 46(26): 12158-12165.
[20] Zhu W G, Zhu M X, Gan Q, Yang Y P, Hu Z Y. Cycolmetalated platinum complexes containing a triarylamine group and used as an electroluminescent material: CN, 196650 [P]. 2007. (in Chinese).
[21] Wu Z L, Zhu M X, Liu Y, Liu J, Li J R, Yang Y P, Gan Q, Zhu W G. Synthesis and photoluminescence of a novel iridium complex (BuPhOXD)2Ir(acac) with unit of 1, 3, 4-oxadiazole [J]. Chin Chem Lett, 2005, 16(2): 241-244.
[22] Deng J Y, Liu Y, Hu Z Y, Zhu M X, Zhu W G. Synthesis and photophysical properties of a new cyclometalated platinum complex containing oxadiazole ligand [J]. J Cent South Univ Technol, 2007, 14(3): 344-347.
(Edited by CHEN Wei-ping)
Foundation item: Projects(20772101, 50473046) supported by the National Natural Science Foundation of China; Project(2007FJ3017) supported by the Hunan Provincial Science Foundation, China; Project(07C764) supported by the Science Foundation of the Education Department of Hunan Province, China
Received date: 2008-08-29; Accepted date: 2008-12-10
Corresponding author: ZHU Wei-guo, Professor, PhD; Tel: +86-732-8298280; Fax: +86-732-8292251; E-mail: zhuwg18@126.com