Electrocatalytic oxidation behavior of L-cysteine at Pt microparticles modified nanofibrous polyaniline film electrode
来源期刊:中南大学学报(英文版)2008年第2期
论文作者:马淞江 罗胜联 周海晖 旷亚非 宁晓辉
文章页码:170 - 170
Key words:electrocatalytic oxidation; electrode; polyaniline; platinum; L-cysteine; nanofibre
Abstract: Platinum(Pt)/nanofibrous polyaniline(PANI) electrode was prepared by pulse galvanostatic method and characterized by scanning electron microscopy. The electrochemical behavior of L-cysteine at the Pt/nanofibrous PANI electrode was investigated by cyclic voltammetry. The results indicate that the pH value of the solution and the Pt loading of the electrode have great effect on the electrocatalytic property of the Pt /nanofibrous PANI electrode; the suitable Pt loading of the electrode is 600 μg/cm2 and the suitable pH value of the solution is 4.5 for investigating L-cysteine oxidation. The L-cysteine sensor based on the Pt/nanofibrous PANI electrode has a good selectivity, reproducibility and stability. The Pt/nanofibrous PANI electrode is highly sensitive to L-cysteine, and the linear calibration curve for the oxidation of L-cysteine can be observed in the range of 0.2-5.0 mmol/L.
基金信息:the PhD. Program Foundation of Ministry of Education of China
the Natural Science Foundation of Hunan Province
the Postdoctoral Foundation of China
J. Cent. South Univ. Technol. (2008) 15: 170-175
DOI: 10.1007/s11771-008-0033-8
![]()
MA Song-jiang(马淞江)1, LUO Sheng-lian(罗胜联)2,
ZHOU Hai-hui(周海晖)2, KUANG Ya-fei(旷亚非)2, NING Xiao-hui(宁晓辉)2
(1.College of Chemistry and Chemical Engineering, Hunan University of Science and Technology,
Xiangtan 411201, China;
2. College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China)
Abstract: Platinum(Pt)/nanofibrous polyaniline(PANI) electrode was prepared by pulse galvanostatic method and characterized by scanning electron microscopy. The electrochemical behavior of L-cysteine at the Pt/nanofibrous PANI electrode was investigated by cyclic voltammetry. The results indicate that the pH value of the solution and the Pt loading of the electrode have great effect on the electrocatalytic property of the Pt /nanofibrous PANI electrode; the suitable Pt loading of the electrode is 600 μg/cm2 and the suitable pH value of the solution is 4.5 for investigating L-cysteine oxidation. The L-cysteine sensor based on the Pt/nanofibrous PANI electrode has a good selectivity, reproducibility and stability. The Pt/nanofibrous PANI electrode is highly sensitive to L-cysteine, and the linear calibration curve for the oxidation of L-cysteine can be observed in the range of 0.2-5.0 mmol/L.
Key words: electrocatalytic oxidation; electrode; polyaniline; platinum; L-cysteine; nanofibre
1 Introduction
Cysteine is an important amino acid on account of its crucial roles in biological systems. For example, it can be used as a prospective radiation protector and cancer indicator[1-2]. So the study on the cysteine oxidation has received widely attention over the world[3-4]. Despite a large number of investigations have been done, there still remain problems to be overcome. One of the main problems is the poor electrochemical response. At ordinary electrodes (Pt, Au, glassy carbon and graphite), the high oxidation potential of cysteine causes the formation of surface oxide, resulting in electrochemical activity of the electrode decreasing dramatically[5]. Consequently, modified electrodes have been employed as a new means to improve the electrochemical oxidation of cysteine. MAZLOUM et al[6] studied the electro- chemical behavior of cysteine at glassy carbon electrode modified with quinizarine. CHEN et al[7] investigated the electrocatalytic oxidation of cysteine at multilayer films of carbon nanotube-modified electrode. They found that the modified electrode showed high electrocatalytic activity toward cysteine. The current was enhanced significantly relative to the situation prevailing when a bare glassy carbon electrode was used and the electrocatalytic process was highly dependent on the pH value of the supporting electrolyte.
On the other hand, polyaniline(PANI), as one of the most important conducting polymer materials, is promising in many fields such as sensor, batteries and electrocatalysis due to the high conductivity, good redox reversibility in air and aqueous solutions[8-11]. Additionally, PANI films modified with Pt microparticles have been used to electro-oxidize methanol[12], glycerol[13] and formic acid[14].
In Ref.[15], nanofibrous PANI films were synthesized on stainless electrode by using pulse gavanostatic method(PGM). The PANI films with nanofibrous structure have more excellent electrochemical properties than granular PANI films. Furthermore, nano-sized Pt particles were electrodeposited onto the surface of the nanofibrous PANI films by PGM. This modified electrode exhibits considerably high electrocatalytic activity for methanol oxidation[16].
In this work, the electrochemical behavior of L-cysteine was investigated by using nanofibrous PANI
film electrode modified with Pt microparticles.
2 Experimental
2.1 Reagent and apparatus
Aniline was purified by repeated distillation and stored under nitrogen gas. All other reagents were of analytical grade. All solutions were prepared with double distilled water. The working electrode was a stainless steel disc with a surface area of 0.08 cm2. The working electrode was polished mechanically using the emery paper (grade 1 000) to a mirror finish and then cleaned with double distilled water in an ultrasonic bath.
All the electrochemical experiments were carried out on a CHI model 660B electrochemical workstation (Shanghai Chenhua Instrument Factory, China) with a three-electrode cell, which consisted of the stainless steel working electrode, a platinum foil counter electrode and a saturated calomel electrode(SCE) as reference electrode at room temperature. All potentials were measured versus SCE.
2.2 Preparation of nanofibrous PANI electrode and Pt/ nanofibrous PANI electrode
The PANI films were synthesized with the pulse galvanostatic system (Handan Instrument Factory, China) by using a two-electrode cell from 0.3 mol/L aniline + 1.0 mol/L HNO3 solution kept at room temperature. The parameters of the pulses used were the ratio of ‘on’ to ‘off’ period (ton?toff)=50 ms?50 ms, mean current density J=1.5 mA/cm2. The thickness of PANI film was 1.25 μm, which was estimated at 800 mC/cm2 [17].
Pt microparticles were electrodeposited on the PANI film by PGM in 7 mmol/L H2PtCl6+0.5 mol/L H2SO4 solution. The PGM deposition was carried out under the following conditions: ton?toff=0.1 ms?9.9 ms, mean current density J = 1.5 mA/cm2.
The electrocatalytic oxidation behavior of L-cysteine at the Pt/nanofibrous PANI electrode was examined in phosphate buffer with 5 mmol/L L-cysteine solution by cyclic voltammetry at 10 mV/s in the scanning range from 0 to 0.7 V. The morphology of the Pt/nanofibrous PANI electrode was characterized by scanning electron microscope (SEM, JSM5600LV).
3 Results and discussion
3.1 Microstructures of Pt/nanofibrous PANI electrode
PANI films with different microstructures can be obtained by different methods. In this work, the PANI films were synthesized by PGM. Fig.1(a) shows the micrograph of the surface of PANI films synthesized by
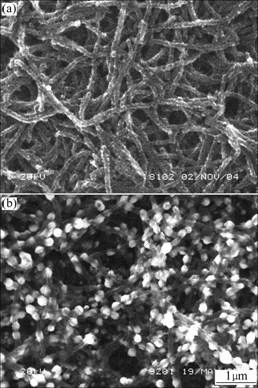
Fig.1 SEM images of nanofibrous PANI electrode (a) and Pt/ nanofibrous PANI electrode (b)
PGM. From Fig.1(a), PANI films with nanofibrous structure can be observed. The fiber has a diameter of 80-120 nm and a length of about 10 μm. Fig.1(b) shows the micrograph of the Pt/nanofibrous PANI electrode. It can be observed that lots of Pt microparticles with a diameter of about 60-120 nm disperse uniformly on the surface of the nanofibrous PANI films. The interesting three-dimensional structure of the Pt/nanofibrous PANI films is beneficial to the dispersion of nano-sized catalyst particles, which may result in outstanding electro- catalytic activity of the Pt/nanofibrous PANI electrode.
3.2 Electrochemical oxidation behavior of L-cysteine on Pt/ nanofibrous PANI electrode
Electrochemical oxidation behavior of L-cysteine on nanofibrous PANI, Pt/nanofibrous PANI and Pt electrodes was investigated by cyclic voltammetry in phosphate buffer with 5 mmol/L L-cysteine solution. Typical cyclic voltammograms are shown in Fig.2. From Fig.2, a broad oxidation peak of L-cysteine can be observed at about 0.6 V on the Pt electrode (curve 2) and no oxidation peak can be observed on the PANI electrode (curve 3). However, a well-defined oxidation peak can be seen and the peak potential shifts in the negative direction on the Pt/nanofibrous PANI electrode (curve 1), which may be due to the synergetic effect of nanofibrous
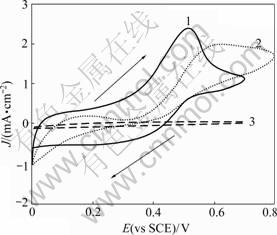
Fig.2 Cyclic voltammograms of different electrodes in phosphate buffer solution (pH = 4.5) with 5 mmol/L L-cysteine at scan rate of 10 mV/s: 1—Pt/nanofibrous PANI electrode; 2—Pt electrode; 3— nanofibrous PANI electrode
PANI film and the Pt microparticles on electrochemical oxidation of L-cysteine. Additionally, the peak current density on the Pt/nanofibrous PANI electrode is about 1.4 times as large as that on the Pt electrode. This implies that the electrochemical oxidation of L-cysteine is improved significantly by Pt/nanofibrous PANI electrode, which may result from the highly dispersed Pt microparticles and the nanofibrous PANI film with high special surface area and good electrochemical properties.
3.3 Effect of Pt loading on electrocatalytic properties of Pt/nanofibrous PANI electrode
The relationship between the forward peak current density(Jp) of cyclic voltammetry and the Pt loading(md) is shown in Fig.3. It can be seen that the forward peak
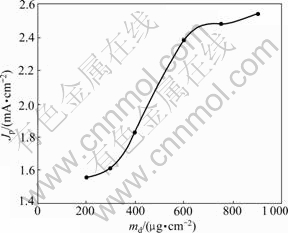
Fig.3 Relationship between peak current density and Pt loading in phosphate buffer solution (pH=4.5) with 5 mmol/L L-cysteine at scan rate of 10 mV/s
current density(Jp) increases linearly with md ranging from 200 to 600 μg/cm2, and then increases slowly after md>600 μg/cm2. The reason may be as follows: when md is low (md<600 μg/cm2), Pt microparticles disperse uniformly on the surface of the nanofibrous PANI film. However, for higher md (md>600 μg/cm2), some Pt microparticles deposited on the surface of the Pt microparticles deposited previously on the PANI film and the accumulation of metal catalyst occurred[18]. Therefore, Jp increases slowly with the further increase of md.
3.4 Effect of pH value on electrocatalytic property of Pt/nanofibrous PANI electrode
The electrochemical oxidation of L-cysteine on the solid electrode may follow the mechanism[19]:
CySH![]() CyS-+H+ (1)
CyS-+H+ (1)
CyS-→![]() +e (2)
+e (2)
2![]() →CySSCy (3)
→CySSCy (3)
It suggests a high pH value dependence of the overall process in the reaction. The dependences of peak potential(Ep) and peak current density(Jp) of L-cysteine oxidation on pH value are shown in Fig.4. As can be seen, a relative high peak current can be observed at pH=4.5, and the L-cysteine oxidation peak shifts to a more positive potential with an increase of pH value, which may be caused by the decrease in conductivity of PANI and the deactivation of L-cysteine with increasing pH value. From Fig.4, it can be obtained that the suitable pH value for investigating L-cysteine oxidation is 4.5, which has been used for subsequent experiments.
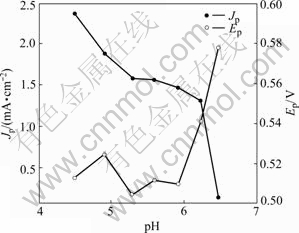
Fig.4 Effects of pH value on peak potential and peak current density of L-cysteine oxidation on Pt/nanofibrous PANI electrode with 5 mmol/L L-cysteine at scan rate of 10 mV/s
3.5 Effect of scan rate on electrocatalytic property of Pt/ nanofibrous PANI electrode
The influences of the scan rate(v) on the peak current density and the peak potential for L-cysteine oxidation were investigated. The corresponding results are shown in Fig.5. It can be observed that the peak potential shifts positively with the increase of the scan rate, and the peak current density increases. At the Pt/nanofibrous PANI electrode, linear relationship between the peak current density and the square root of the scan rate can be obtained (Fig.5(a)). This implies that the reaction of L-cysteine oxidation may be controlled by a diffusion process. Fig.5(b) shows the dependence of the peak potential for L-cysteine oxidation reaction upon lg v. As an irreversible oxidation process, the peak potential can be presented by the equation[20]:
![]() (4)
(4)
where A is a constant, which is related to the standard electrode potential(E0) and standard rate constant at E0.

Fig.5 Plots of peak current density (a) and peak potential (b) of cysteine oxidation at Pt/nanofibrous PANI electrode against square root of scan rate(v1/2) and logarithm of scan rate(v)
The transfer coefficient(α), which characterizes the effect of electrochemical potential on activation energy of an electrochemical reaction, can be calculated according to the slope of Ep-lg v. From Fig.5(b), the value is calculated to be 0.61 (n=2) for the Pt/nanofibrous PANI electrode.
3.6 Analytical property of Pt/nanofibrous PANI electrode in detecting L-cysteine
The Pt/nanofibrous PANI electrode was used to detect the concentration of L-cysteine. The current response of the Pt/nanofibrous PANI electrode to L-cysteine is shown in Fig.6(a). It is clear that the Pt/nanofibrous PANI electrode has good current response to the addition of L-cysteine, and a plateau with a large response current can be obtained obviously. This indicates that the Pt/nanofibrous PANI electrode is highly sensitive to L-cysteine. The dependence of the
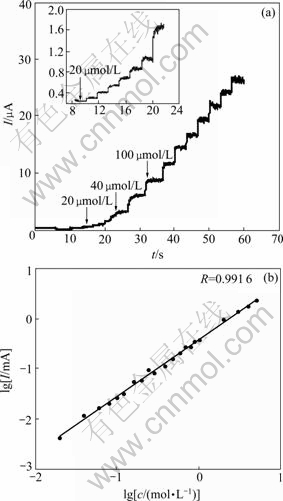
Fig.6 Anodic current response of Pt/nanofibrous PANI electrode to addition (indicated by arrows) of various concentrations of L-cysteine in phosphate buffer solution (pH= 4.5) at applied potential of 0.5 V (a) and the calibration curve for current vs L-cysteine concentration (b)
steady-state response current on the concentration of L-cysteine is shown in Fig.6(b). It can be seen that lg I of L-cysteine oxidation at the Pt/nanofibrous PANI electrode increases linearly with lgc and can be expressed as lg I=1.127lg c-0.362 6. The responses are reproducible from electrode to electrode and from day to day for freshly prepared electrodes.
Fig.7 shows the amperometric response of the Pt/ nanofibrous PANI electrode to three oxidizable amino acids at 0.5 V. From Fig.7, the contributions of tryptophan (20 μmol/L, B) and tyrosine (20 μmol/L, C) are nearly eliminated at the Pt/nanofibrous PANI electrode. This implies that the Pt/nanofibrous PANI electrode has a good selectivity for L-cysteine.
The reproducibility and stability of the Pt/ nanofibrous PANI electrode were also tested. The reproducibility experiments were run at eight Pt/ nanofibrous PANI electrodes. The results demonstrate that the Pt/nanofibrous PANI electrode has a good reproducibility with a relative standard deviation of 6.54%. And the electrocatalytic property of the Pt/nanofibrous PANI electrode to L-cysteine oxidation remains satisfactory, only 10% current loss after 7 d and 20% current loss after 30 d are observed. This indicates that the Pt/nanofibrous PANI electrode can be used as L-cysteine sensor.
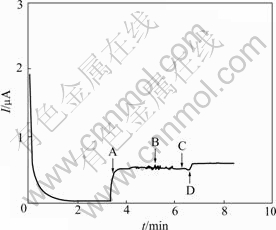
Fig.7 Anodic current response of Pt/nanofibrous PANI electrode to additions (indicated by arrows) of 100 μmol L-cysteine (A), 20 μmol tyrosine (B), 20 μmol tryptophan (C) and 20 μmol L-cysteine (D) respectively in phosphate buffer solution (pH=4.5) at applied potential of 0.5 V
4 Conclusions
1) Pt particles modified nanofibrous PANI electrode is prepared by PGM. Effects of Pt loading of the electrode and pH value of the solution on electrocatalytic property of the Pt/nanofibrous PANI electrode are also investigated. The optimal Pt loading and pH value is 600 μg/cm2 and 4.5.
2) Electrochemical oxidation behavior of L-cysteine at the Pt/nanofibrous PANI electrode is studied. Compared with Pt electrode, the oxidation peak potential is more negative and the oxidation peak current density is larger for the Pt/nanofibrous PANI electrode, which suggests that the electrochemical oxidation behavior of L-cysteine is improved greatly by using the Pt/nanofibrous PANI electrode.
3) The Pt/nanofibrous PANI electrode exhibits good sensitivity, selectivity, reproducibility and stability. The linear response range of the electrode for L-cysteine is 0.2-5.0 mmol/L, which indicates that the electrode can be used to detect L-cysteine after further research.
References
[1] SANG Y, HEE J, EUNSOOK N, JONG C, CHAEHWA P. Expression of reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) as a prognostic indicator in gastric cancer[J]. European Journal of Cancer,2006, 42(1): 101-108.
[2] PAOLA C, CONCETTA D, OTTAVIA G, SILVIO S. Thermodynamic and spectroscopic study for the interaction of dimethyltin (IV) with L-cysteine![]() in aqueous solution[J]. Biophysical Chemistry, 2008, 133(1/3): 19-27.
in aqueous solution[J]. Biophysical Chemistry, 2008, 133(1/3): 19-27.
[3] MALEKI N, SAAFAVI A, SEDAGHATI F, TAJABADI F. Efficient electrocatalysis of L-cysteine oxidation at carbon ionic liquid electrode[J]. Analytical Biochemistry, 2007, 369(2): 149-153.
[4] NTHAPO S, TEBELLO N. Electrocatalytic oxidation of thiocyanate, l-cysteine and 2-mercaptoethanol by self-assembled monolayer of cobalt tetraethoxy thiophene phthalocyanine[J]. Electrochimica Acta, 2006, 51(21): 4463-4470.
[5] FAWCETT W, FEDURCO M, KOVACOVA Z. Oxidation of cysteine cysteinesulfinic acid and cysteic acid on a polycrystalline gold electrode[J]. Journal of Electroanalytical Chemistry, 1994, 368(1/2): 265-274.
[6] MAZLOUM M, RAHIMI P, EBRAHIMI P, HAMID R, NAEIMI H. Electrocatalytic oxidation of cysteine by quinizarine at glassy carbon electrode[J]. Sensors and Actuators B: Chemical, 2007, 123(2): 763-768.
[7] CHEN Xu, YANG Yi, DING Ming-yu. Electrocatalytic oxidation and sensitive detection of cysteine at layer-by-layer assembled carbon nanotube-modified electrode[J]. Analytica Chimica Acta,2006, 557(1/2): 52-56.
[8] WANG Z, YUAN J, LI M, IVASKA A. Electropolymerization and catalysis of well-dispersed polyaniline/carbon nanotube/gold composite[J]. Journal of Electroanalytical Chemistry,2007, 599(1): 121-126.
[9] ZHOU Hai-hui, CHEN Hong, CHEN Jin-hua, KUANG Ya-fei. Polyaniline-graphite composite film glucose oxidase electrode[J]. Journal of Central South University of Technology, 2006, 13(6): 653-657.
[10] KHADIJEH G, MIR F, MOJTABA S, HASSAN K. Synthesis of polyaniline/graphite composite as a cathode of Zn-polyaniline rechargeable battery[J]. Journal of Power Sources,2007, 170(2): 513-519.
[11] LAI Yan-qing, LI Jing, LI Jie, LU Hai, ZHANG Zhi-an, LIU Ye-xiang. Preparation and electrochemical characterization of C/PANI composite electrode materials[J]. Journal of Central South University of Technology, 2006, 13(4): 353-359.
[12] HU Z, REN L, FENG X, WANG Y, YANG Y, LEI Z. Platinum-modified polyaniline/polysulfone composite film electrodes and their electrocatalytic activity for methanol oxidation[J]. Electrochemistry Communications,2007, 9(1): 97-102.
[13] NIRMALA A, PANDIAN K. Pt, Pt-Pd and Pt-Pd/Ru nanoparticles entrapped polyaniline electrodes—A potent electrocatalyst towards the oxidation of glycerol[J]. Electrochemistry Communications,2006, 8(8): 1340-1348.
[14] ZHU Zan-zan, WANG Zhe, LI Hu-lin. Functional multi-walled carbon nanotube/polyaniline composite films as supports of platinum for formic acid electrooxidation[J]. Applied Surface Science,2008, 254(10): 2934-2940.
[15] ZHOU Hai-hui, JIAO Shu-qiang, CHEN Jin-hua, WEI Wan-zhi, KUANG Ya-fei. Relationship between preparation conditions, morphology and electrochemical properties of polyaniline prepared by pulse galvanostatic method[J]. Thin Solid Films, 2004, 450(2): 233-239.
[16] ZHOU Hai-hui, JIAO Shu-qiang, CHEN Jin-hua, WEI Wan-zhi, KUANG Ya-fei. Effects of conductive polyaniline preparation and platinum electrodeposition on electroactivity of methanol oxidation[J]. Journal of Applied Electrochemistry, 2004, 34(4): 455-459.
[17] DIN H, BIRSS V. Electrochemical and mass measurements during small voltage amplitude perturbations of conducting polyaniline films[J]. Journal of Electroanalytical Chemistry, 1998, 443(1): 63-71.
[18] YE J, FEDKIW P. Electrodeposition of high-surface area platinum in a well adherent nafion film on glassy carbon[J]. Electrochimica Acta,1996, 41(2): 221-231.
[19] RALPH T, HITCHMAN M, MILLINGTON J, WALSH F. The electrochemistry of L-cystine and L-cysteine (Part 1): Thermodynamic and kinetic studies[J]. Journal of Electroanalytical Chemistry, 1994, 375(1/2): 1-15.
[20] LAVIRON E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems[J]. Journal of Electroanalytical Chemistry, 1979, 101(1): 19-28.
(Edited by CHEN Wei-ping)
Foundation item: Project(20050532008) supported by the PhD. Program Foundation of Ministry of Education of China; Project(06JJ4005) supported by the Natural Science Foundation of Hunan Province; Project(20060400874)supported by the Postdoctoral Foundation of China; Project supported by the Postdoctoral Foundation of Hunan University
Received date: 2007-08-26; Accepted date: 2007-09-30
Corresponding author: MA Song-jiang, Senior Engineer; Tel: +86-732-8383072; E-mail: masji@sohu.com

