合金元素对铝阳极电化学性能的影响
张纯,王日初,冯艳,邱科,彭超群
(中南大学 材料科学与工程学院,湖南 长沙,410083)
摘要:通过正交试验法研究Mg,Sn和Hg元素对铝合金阳极电化学性能的影响,采用恒电流扫描法、动电位极化法和排水析氢法检测Al-Mg-Sn-Hg阳极的电化学性能和腐蚀性能,采用环境扫描电镜结合能谱分析研究Al-Mg-Sn-Hg阳极的表面形貌和显微组织。结果表明:Sn和Hg的加入使得一部分Mg与2种元素形成第二相,成为80 ℃、大电流密度下铝阳极活化的主要因素,并能很好地抑制析氢;另一部分Mg固溶在Al中,成为铝阳极保持活化状态的重要原因;得到了综合性能优良的Al-Mg-Sn-Hg铝合金阳极材料,它在电流密度为650 mA/cm2、80 ℃、4.5%的NaOH溶液中稳定电位为-1.707 V,析氢速率为0.38 mL/(cm2·min)。
关键词:铝合金阳极;电化学性能;析氢;正交试验
中图分类号:TG146.2+1 文献标志码:A 文章编号:1672-7207(2012)01-0081-06
Effects of alloying elements on electrochemical performance of aluminum anodes
ZHANG Chun, WANG Ri-chu, FENG Yan, QIU Ke, PENG Chao-qun
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: An Al-Mg-Sn-Hg alloy anode material was developed by orthogonally design to analyze the effects of additive elements on performance of Al alloy anode. Its electrochemical properties in alkaline medium were studied by galvanostatic test and potentiodynamic potential test. The corrosion resistance of Al alloy anode was studied by hydrogen evolution. The effects of additive elements on phase composition and microstructure of Al anode were studied by X-ray diffraction, scanning electron microscopy and energy dispersive spectroscopy. The results show that a part of Mg can form the second phase with Sn and Hg, which is the main cause of activating the aluminum at high temperature and large current density. Another part of Mg which solves in aluminum is an important factor to maintain aluminum activate. The stable potential of new developed Al-Mg-Sn-Hg anode is -1.707 V and the rate of hydrogen evolution is 0.38 mL/(cm2·min) at 80 ℃, 650 mA/cm2 and 4.5% NaOH.
Key words: aluminum anode; electrochemical performance; hydrogen evolution; orthogonal test
动力电池性能的提高对鱼雷航速和航程的提高起决定作用。为了提高比功率和比能量,常常使用电极电位低的活泼金属材料作阳极[1]。Al的电化学当量为2.98 (A·h)/g,体积当量为8.10 (A·h)/cm3,是除Li外质量比能量最高的金属[2]。Al的来源广泛,储量丰富,价格低廉,是一种理想的阳极材料。但是,Al在空气或水溶液中易自钝化,使其稳定的工作电位(vs Hg/HgO)仅为-0.74 V [ 3]。在碱性溶液中氧化膜破坏,电位降低,自腐蚀较大,析氢量增加,电流效率较低,导致铝阳极的法拉第效率降低[4]。通过添加合金元素Mg,Pb,In,Bi,Hg,Ga,Sn和Zn等[5-9]减少铝阳极的阳极极化,降低其自腐蚀速率,提高电极的利用率,是活化铝阳极最有效的方法之一。游文等[10]开发了Al-Ga-In-Zn-Mg-Mn阳极,在80 ℃,电流密度为800 mA/cm2条件下,在添加偏铝酸盐的4 mol/L NaOH溶液中的稳定电位(vs Hg/HgO)为-1.576 V。马正青 等[11-12]也开发出一系列在碱性溶液中具有良好电化学性能的铝阳极。虽然国内对铝合金阳极材料已经进行了不少研究,但是,随着动力电池对铝阳极电池性能要求的不断提高,进一步研究在高温条件下工作的铝阳极材料,尤其是大电流密度工作条件下的铝阳极材料具有重要的理论意义和实用价值。本文通过正交试验研究Mg,Sn和Hg 3种合金元素对铝阳极材料的电化学、腐蚀性能和阳极极化行为的影响,优化出铝合金阳极的成分。该成分合金在电流密度为 650 mA/cm2、温度为80 ℃、NaOH质量分数为4.5%的溶液中具有优良的综合性能。
1 实验
1.1 合金的制备
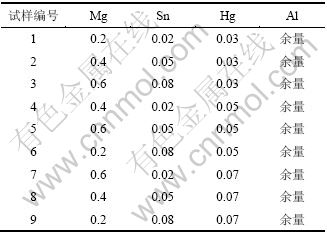
采用正交表L9(34)设计Al-Mg-Sn-Hg中的元素名义成分(如表1所示)。合金采用井式炉、石墨坩埚熔炼,其中Al和Mg以纯金属加入,Hg以Mg-20%Hg(质量分数)中间合金,Sn以Al-10%Sn(质量分数)中间合金形式加入,熔炼温度为750 ℃,加覆盖剂和除气剂,石墨棒搅拌静置后铁模浇注成型。刨面后轧制成 0.5 mm厚的板材。
表1 铝合金阳极各试样成分(质量分数)的正交设计
Table 1 Orthogonal design for chemical compositions of Al anode materials %
1.2 电化学性能测定
将试样剪切成方形,与铜线相连,留一面作为工作面,非工作面用环氧树脂密封,工作面用金相砂纸逐级打磨至800#砂纸,用无水乙醇超声波清洗并干燥。在4.5% NaOH溶液中,采用3电极系统(如图1所示),以铝合金样品为工作电极,铂片为辅助电极,饱和甘汞电极为参比电极,采用IM6EX型电化学综合测试仪,在80 ℃恒温水浴中测试不同成分铝合金样品的恒电流曲线(电流密度650 mA/cm2,持续时间1 000 s)和动电位扫描曲线(扫描速度5 mV/s,扫描范围-2.4~-1.5 V),获得铝合金阳极的稳定电位、开路电位和自腐蚀电流密度。采用排水法测定样品的析氢速率。
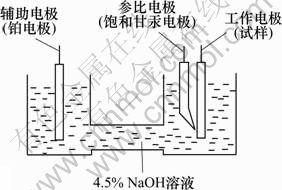
图1 电化学实验装置图
Fig.1 Setting diagram of electrochemical experiment
1.3 铝阳极微观组织和腐蚀形貌观察
对铝阳极材料进行金相砂纸预磨后抛光,采用 JSM-5600Lv 扫描电子显微镜观察铝合金阳极材料表面形貌,采用能谱扫描确定第二相的成分。
2 结果与讨论
2.1 合金元素对铝合金阳极电化学性能的影响
铝合金阳极各试样的稳定电位、腐蚀电流密度、开路电位和析氢速率如表2所示。从表2可以看出:试样7和9的稳定电位均较负,分别为-1.707 V和-1.690 V。试样1的稳定电位最正,仅为-1.204 V。图2(a)所示为试样2,4,7和9的恒电流检测曲线,可以看出:试样2和4放电曲线比较平滑,无剧烈起伏,试样7在前100 s中有较小波动,但维持在-1.75 V左右,100 s以后曲线趋于平滑,说明在放电过程中表现出较好的电化学活性,合金在整个放电过程中都处于较好的活化状态;试样9在整个放电过程中稳定性相对较差,说明在放电过程中腐蚀产物的剥落不均匀,使其在某些点出现轻微的极化现象。图2(b)所示为铝合金阳极的试样2,4,7和9的动电位极化扫描曲线,可以看出:随着极化电位的正移,铝合金阳极的腐蚀电流迅速增加,一直表现为阳极溶解的过程,没有出现钝化,说明4种铝合金阳极在80 ℃和650 mA/cm2放电条件下,具有较高的电化学活性。因此,Mg、Sn和Hg 3种合金元素的加入都能对铝阳极起到一定的活化作用,使得铝阳极在放电过程中一直表现出活化极化控制。
表2 铝合金阳极各试样的稳定电位、腐蚀电流密度、开路电位和析氢速率
Table 2 Stable potentials, corrosion current densities, open circuit potential and rate of hydrogen evolution of Al anode material specimens
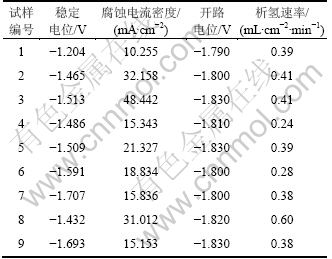
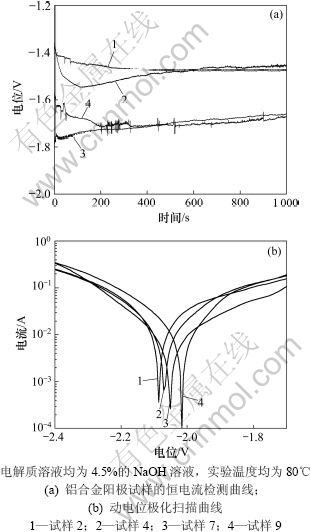
图2 铝合金阳极试样的恒电流检测曲线和动电位极化扫描曲线
Fig.2 Galvanostatic curves(a) and polarization curves(b) of Al anode material specimens
铝合金阳极合金元素的成分是通过正交设计获得的(如表1所示)。表3所示为铝合金阳极的稳定电位、析氢速率、腐蚀电流密度和开路电位正交实验分析各因素影响主次的结果。表4所示为各个因素主次及其较优水平。从影响稳定电位的因素的较优水平也可以得出,影响铝合金阳极的稳定电位因素中,Hg的影响最大,Hg含量越高,稳定电位越负。从表4所列中影响腐蚀电流密度的因素主次来看,Sn是主要因素。因为由于Mg和Sn匹配能形成Mg2Sn第二相[13],并且在铝阳极中均匀分布,使铝阳极在高温沉积作用减弱的情况下,放电过程中形成较多的活性点,从而破坏铝阳极表面形成的钝化膜连续性,并且由于这种弥散的分布使这种活化作用呈持续性,从而使其均匀腐蚀。但是Sn容易在晶界形成偏析相,富锡相容易使晶界优先腐蚀,增大铝阳极的自腐蚀,所以,铝阳极中Sn元素的加入不宜过多;固溶在Al基体中的Mg是铝阳极均匀腐蚀的原因之一。
表3 稳定电位、腐蚀电流密度、析氢速率、开路电位的正交试验结果
Table 3 Results of orthogonal tests of stable potentials, corrosion current densities, open circuit potential, hydrogen evolution
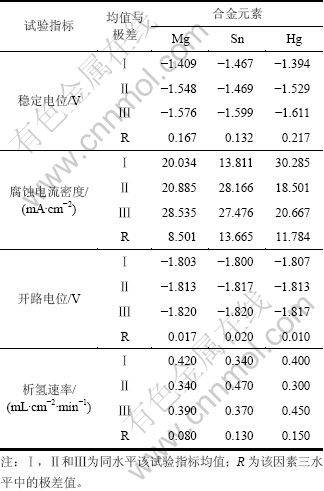
表4 影响铝合金阳极稳定电位、腐蚀电流密度和析氢速率的因素主次及其较优水平
Table 4 Sequence of elements and optimized level of stable potentials, open circuit potential, corrosion current density and rate of hydrogen evolution of Al anode material specimens

从上述分析可知:Hg元素不仅是优良的活化元素,而且析氢速率的影响因素中Hg的影响最大,从而适量的Hg可以明显降低铝阳极腐蚀过程中伴随的析氢速率。由于适量的Mg,Sn与Hg作用的结果,使得铝合金阳极在80 ℃,电流密度为650 mA/cm2条件下的4.5% NaOH溶液中,活化性能的提高与自腐蚀速率的降低得到平衡、稳定控制,从而具有优良的综合电化学性能。
2.2 合金元素对铝阳极显微组织的影响
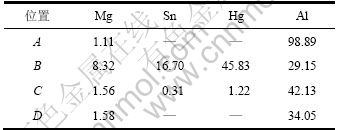
为了满足大功率海水电池的要求,现代鱼雷动力电池的发展趋势要求负极在能承受大电流密度的同时具有很负的电极电位[1]。在提高铝的活性的同时,必须有效抑制其析氢速率增加,才能保证铝阳极的电流效率。因此,根据综合平衡原则,考虑3种合金元素对以上4个指标的影响,一般选取稳定电位和析氢速率为主要因素,其次是开路电位和腐蚀电流密度。从以上的正交实验分析可知:最佳的合金成分水平为Mg3Sn1Hg3,即正交表中试样7的合金成分,从而选取试样7的合金作为研究对象。铝合金阳极在腐蚀过程中要求稳定的电化学活性、腐蚀表面均匀腐蚀、腐蚀产物易于剥落。图3(a)所示为试样7的表面形貌,可以看出铝阳极中均匀分布着细小的第二相,它们是降低铝阳极的自腐蚀速率的原因之一[14]。结合表5中的能谱分析结果可以得出:基体为Al和Mg 2种合金元素。Mg是化学活性很高的金属,当镁的质量分数小于1%时,Mg基本固溶在Al中[15],但由于Hg和Sn的存在,使得一部分Mg与Hg和Sn形成第二相。这些第二相是点蚀萌生的敏感部位,使铝阳极在高温条件下腐蚀产物黏附力变强,在合金元素沉积作用减弱的的情况下,仍然以偏析相优先溶解的方式连续地活化铝阳极。
从试样7合金的腐蚀后表面形貌(如图4(a)所示) 可以看出:合金腐蚀表面有鳞片状、白色絮状腐蚀产物,以及片状脱落以后留下的新鲜金属表面。这说明腐蚀产物在80℃的4.5%NaOH溶液中与基体的附着力较差,易于从试样表面剥落,使得Al基体裸露出来,从而使铝阳极在整个放电过程中维持较好的活化状态。从图4(b)所示的析氢后断面腐蚀的形貌可以看出:腐蚀是从点蚀开始的,但点蚀深度比较小,只破坏了氧化膜,氧化膜被破坏以后整个试样表面开始腐蚀,说明是均匀腐蚀的,因此,使得整个铝阳极板在工作过程中不至于穿孔而导致电流效率下降。结合能谱分析可知,发生点蚀的主要原因是Hg在Al中的溶解度小,只能和Mg形成第二相。这些第二相的均匀分布破坏了铝阳极表面的氧化膜的致密性和连续性。在合金溶解初期,与Al基体耦合造成点蚀,破坏了表面氧化膜的结构,致使Al基体裸露出来,从而使Al得到活化。由于Sn形成的高氢超电位第二相[16]的存在,使铝阳极不仅得到活化,而且其伴随的析氢反应也得到抑制。由于Al基体中固溶的Mg,Hg和Sn的溶解再沉积,使得铝阳极在整个放电过程中都可以维持稳定的工作状态,并且沉积的Hg和Sn等高氢过电位元素[17]富集在阴极相上,特别是低熔点的Hg上,使得整个工作过程中的析氢反应一直得到稳定抑制[18],从而铝阳极能在工作过程中表现出稳定的综合电化学性能。综上所述,由于铝阳极中添加的3种合金元素的综合作用,试样7在整个放电过程中表现出优良的电化学性能。通过添加适量的合金元素Mg,Sn和Hg,不仅能使铝阳极在高温、大电流密度的条件下具有较负的稳定电位和较小的析氢速率,而且使铝合金阳极在整个腐蚀过程中维持较优的电化学活性,腐蚀产物容易剥落,从而腐蚀均匀。
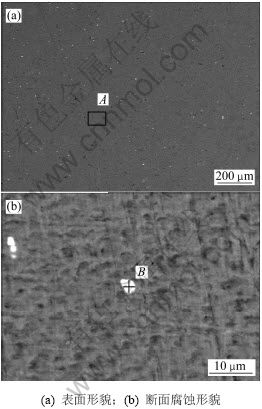
图3 试样7腐蚀前扫描电镜照片
Fig.3 SEM images of Specimen 7
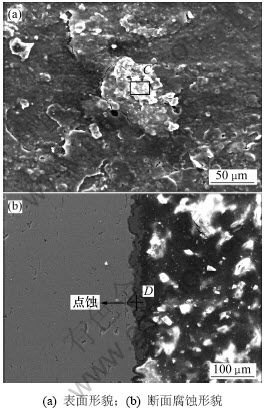
图4 试样7析氢腐蚀后的表面形貌和试样断面腐蚀形貌
Fig.4 SEM surface morphology of corroded surface and fracture surface morphology of corroded surface of Specimen 7
表5 图3和图4中各点的能谱成分分析(质量分数)
Table 5 Chemical composition of different points in Figs.3 and 4 %
3 结论
(1) 在碱性高温环境中,Hg是铝阳极活化和抑制析氢速率的优良元素,Sn是影响耐蚀性的主要因素,过量的Sn增大铝阳极的自腐蚀速率;Mg是铝阳极在放电过程中维持均匀腐蚀的重要因素。
(2) Sn,Hg与Mg形成的第二相是高温、大电流密度下铝阳极激活的主要因素,并且通过Mg,Sn和Hg 3种元素的溶解再沉积作用,能很好地抑制析氢速率,使铝合金阳极的活化性能的提高与析氢速率的降低达到一个平衡稳定的状态。
(3) 通过正交实验得出一个具有良好性能的Al-Mg-Sn-Hg阳极材料,在4.5% NaOH溶液中开路电位为-1.800 V,稳定电位为-1.707 V,自腐蚀速率为15.836 mA/cm2,析氢速率为0.38 mL/(cm2·min),并且腐蚀产物容易剥落,腐蚀均匀。
参考文献:
[1] 蔡年生. 国外鱼雷动力电池的发展及应用[J]. 鱼雷技术, 2003, 11(1): 12-16.
CAI Nian-sheng. Development and application of batteries for overseas torpedo propulsion[J]. Torpedo Technology, 2003, 11(1): 12-16.
[2] 林顺岩, 田士, 游文. 电池用铝合金阳极材料研究的新进展[J]. 铝加工, 2006, 6(172): 11-14.
LING Shun-yan, TIAN Shi, YOU Wen. Recent development of aluminum alloy material for battery anodes[J]. Aluminium Fabrication, 2006, 6(172): 11-14.
[3] Kalaignan G P, Muthuramalingam R. Studies of electrochemical behavior of aluminium and its alloys in alkaline media[J]. Bulletin of Electrochemistry, 1999, 15(1): 42-46.
[4] 鞠克江, 刘长瑞, 唐长斌, 等. 铝空气电池的研究进展及应用前[J]. 电池, 2009, 39(1): 50-52.
JU Ke-jiang, LIU Chang-rui, TANG Chang-bin, et al. Research progress and application prospects of aluminum air batteries[J]. Battery Bimonthly, 2009, 39(1): 50-52.
[5] Flamini D O, Saidman S B, Bessone J B. Aluminium activation produced by gallium[J]. Corrosion Science, 2006, 48: 1413-1425.
[6] Bessone J B, Flamini D O. Comprehensive model for the activation mechanism of Al-Zn alloys produced by indium[J]. Corrosion Science, 2005, 47: 95-105.
[7] Shibli S M A, Jabeera B, Manu R. Development of high performance aluminium alloy sacrificial anodes reinforced with metal oxides[J]. Materials Letters, 2007, 61(14/15): 3000-3004.
[8] MA Jing-ling, WEN Jiu-ba. Influence of Mg and Ti on the microstructure and electrochemical performance of aluminum alloy sacrificial anodes[J]. Rare Metals, 2009, 28(2): 187-192.
[9] Bessone J B. The activation of aluminum by mercury ions in non-aggressive media[J]. Corrosion Science, 2006, 48(5): 42-43.
[10] 游文, 林顺岩. 新型铝合金阳极在NaOH碱性溶液中的腐蚀行为[J]. 铝加工, 2006(3): 15-18.
YOU Wen, LIN Shun-yan. Corrosion behaviour of new aluminum alloy anode in NaOH alkaline solution[J]. Aluminium Fabrication, 2006(3): 15-18.
[11] 马正青, 曾苏民, 黎文献. 高活性铝合金负极材料的电化学性能研究[J]. 表面技术, 2005, 34(1): 25-33.
MA Zheng-qing, ZENG Su-min, LI Wen-xian. Study on electrochemical performance of aluminum alloys anode materials[J]. Surface Technology, 2005, 34(1): 25-33.
[12] 尹延西, 李卿, 江洪林, 等. 高性能铝合金阳极碱性介质中的电化学性能[J]. 稀有金属材料与工程, 2009, 38(增刊1): 76-79.
YIN Yan-xi, LI Qing, JIANG Hong-lin, et al. Electrochemical performances of aluminum alloy anodes in alkaline solution[J]. Rare Metal Materials and Engineering, 2009, 38(Suppl 1): 76-79.
[13] Sameljuk A V, Neikov O D, Krajnikov A V, et al. Effect of rapid solidification on the microstructure and corrosion behaviour of Al-Zn-Mg based material[J]. Corrosion Science, 2007, 49(2): 276-286.
[14] Kim H, Kim Y J, Kim D G, et al. Mechanochemical synthesis and electrochemical characteristics of Mg2Sn as an anode material for Li-ion batteries[J]. Solid State Ionics, 2001, 144(1/2): 41-49.
[15] 林顺岩, 田士. 新型铝合金阳极的退火处理工艺[J]. 热加工工艺, 2006, 35(18): 50-51.
LING Shun-yan, TIAN Shi. Annealing technology of new aluminum alloy anodes[J]. Material and Heat Treatment, 2006, 35(18): 50-51.
[16] 齐公台, 郭稚弧, 屈钧娥. 合金元素Mg对含RE铝阳极组织与性能的影响[J]. 中国腐蚀与防护学报, 2001, 21(4): 220-224.
QI Gong-tai, GUO Zhi-hu, QU Jun-e. Effect of magnesium on microstructure and performance of containing RE aluminum anode[J]. Journal of Chinese Society for Corrosion and Protection, 2001, 21(4): 220-224.
[17] Sina N, Emamy M, Saremi M. The influence of Ti and Zr on electrochemical properties of aluminum sacrificial anodes[J]. Materials Science and Engineering, 2006, 431(1/2): 263-276.
[18] 余祖孝, 郝世雄, 龚敏. 微量HgCl2对铝阳极电化学行为的影响[J]. 电池, 2005, 35(5): 368-370.
YU Zu-xiao, HAO Shi-xiong, GONG Min. Effect of trace HgCl2 on the electrochemical behavior of aluminum anode[J]. Battery Bimionthly, 2005, 35(5): 368-370.
(编辑 邓履翔)
收稿日期:2011-01-23;修回日期:2011-03-28
基金项目:国家自然科学基金资助项目(51101171)
通信作者:王日初(1965-),男,广东和平人,博士,教授,从事金属及粉末冶金材料研究;电话:0731-88836638;E-mail: wrc@csu.edu.cn