DOI: 10.11817/j.issn.1672-7207.2018.12.002
含钛钢渣在熔融NaOH体系中焙烧过程及其反应机理
赵昌明1,宁哲1,张崇民1,蔡永红1, 2,王一雍1,邵品1,李继东1
(1. 辽宁科技大学 材料与冶金学院,辽宁 鞍山,114051;
2. 攀钢集团 成都钢钒有限公司,四川 攀枝花,617000)
摘要:针对高钙、高磷型含钛钢渣的特点,采用NaOH为焙烧剂,考察焙烧温度、焙烧时间、碱矿比对含钛钢渣在熔融NaOH体系中TiO2提取率的影响,并对焙烧过程动力学进行解析。研究结果表明:含钛钢渣在熔融NaOH体系中优化反应条件为焙烧温度450 ℃,碱矿比6:1,焙烧时间60 min;当焙烧温度为350~500 ℃时,含钛钢渣在熔融NaOH体系中焙烧过程由内扩散控制,反应表观活化能E=10.77 kJ/mol,其动力学方程可描述为:1-2X/3-(1-X)2/3=1.239exp[-39.4/(RT)]t;含钛钢渣中主要物相Mg2TiO4,MnTi2O4和CaTiO3在熔融NaOH体系中焙烧过程中已完全分解,为后续钛的提取创造了有利条件。
关键词:含钛钢渣;熔融;动力学;焙烧
中图分类号:TF09 文献标志码:A 文章编号:1672-7207(2018)12-2908-07
Reaction mechanism of titanium-bearing steel slag roasting in NaOH melt
ZHAO Changming1, NING Zhe1, ZHANG Chongmin1, CAI Yonghong1, 2,WANG Yiyong1, SHAO Pin1, LI Jidong1
(1. School of Materials and Metallurgy, University of Science and Technology Liaoning, Anshan 114051, China;
2. Chengdu Iron & Steel Co., Ltd., Pangang Group, Panzhihua 617000, China)
Abstract: With consideration of the high content of calcium and phosphorus, titanium-bearing steel slag was roasted with NaOH to investigate the effects of roasting temperature, roasting time and mass ration of alkali-to-ore on the TiO2 extraction rate. The kinetics of the roasting process were analyzed. The results show that the optimal conditions of titanium-bearing steel slag reacted with NaOH melt are as follows: roasting temperature 450 ℃, alkali-to-ore ratio 6:1 and roasting time 60 min. The roasting process is found to be diffusion controlled at temperature of 350-500 ℃. The apparent activation energy is 10.77 kJ/mol, and the kinetic equation can be described as follows: 1-2X/3-(1-X)2/3= 1.239exp[-39.4/(RT)]t. The complete decomposition of the main phases (Mg2TiO4, MnTi2O4, CaTiO3) in titanium-bearing steel slag after roasting with NaOH has created favorable conditions for the extraction of titanium.
Key words: titanium-bearing steel slag; melting; kinetics; roasting
钛是一种重要的战略储备资源。钛及其产品具有优良的物理化学特性,如耐高温、抗腐蚀及高强度性,被广泛应用在军工、机械、航空航天等领域[1-4]。近年来,随着攀西地区磁铁矿资源开发利用的逐渐深入,攀成钢等企业也逐步使用钒钛磁铁矿替代普通铁矿石进行冶炼;随生产规模逐渐扩大,这些生产企业每年将产生数百万吨的高钙、高磷型含钛钢渣,该含钛渣与其他含钛渣相比赋存物相复杂,矿物结晶微细、渣中含钛化合物被以硅酸盐为主的辉石相所包裹,若采用浮选分离或改性处理都较困难[5-8]。同时,大量含钛钢渣堆放造成钛资源浪费以及土壤、水域污染。为此,需要研发一种清洁、高效的利用工艺,为我国大量堆积的含钛钢渣找到合理的利用途径。近年来,针对含钛钢渣的综合利用,国内外研究者开展了大量的研究工作并取得了一定的研究成果。HE等[9]采用50%(质量分数)的稀硫酸处理含钛渣获得富钛产品,TiO2浸出率为82%,浸出过程为内扩散控制;熊瑶等[10]研究不同粒径的含钛渣在20%盐酸溶液中的浸出行为,TiO2 浸出率达72%;隋丽丽等[11]采用浓硫酸焙烧高钛渣制备TiO2,并对焙烧过程动力学进行解析。酸法处理含钛渣需要较高浓度的酸液处理,不仅造成废酸的二次污染,而且酸浸残渣更难以利用。WU等[12]通过高温变质、重选和湿法冶金等方法,从含钛渣中提取钛和金属铁,并研究金属铁在高温变质过程中的行为及从基体相中分离金红石的可行性,但由于选矿技术等原因导致分离效果不理想。本文作者基于现有含钛钢渣利用工艺的研究现状,结合高钙、高磷型含钛钢渣的特点,采用NaOH为焙烧剂,考察焙烧温度、焙烧时间、碱矿比对含钛钢渣在熔融NaOH体系中TiO2提取率的影响,并对焙烧过程动力学进行解析。
1 实验部分
1.1 实验原料与试剂
实验所用含钛钢渣来自攀成钢。首先,将含钛钢渣用制样机破碎至0.8~1.0 mm的粒度,然后放入干燥箱内恒温烘干2 h后备用。取一部分含钛钢渣进行研磨筛分(粒度小于0.074 mm),其化学成分见表1。由表1可知:渣中主要元素为 V,Ti,Si,Ca,Fe,Mn等,其中CaO和P2O5含量高,且CaO与TiO2质量比接近3,属于高钙、高磷型含钛渣;X线衍射分析(XRD)和扫面电镜分别如图1和图2所示,含钛钢渣表面成不规则形态,质地坚硬,主要赋存物相由尖晶石相(FeV2O4),钛晶石相(Mg2TiO4)、(MnTi2O4)和(CaTiO3),辉石相(CaMgSi2O6)组成。
表1 含钛渣的化学成分(质量分数)
Table 1 Chemical composition of titanium slag %
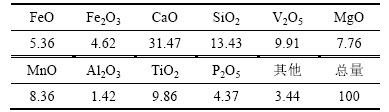
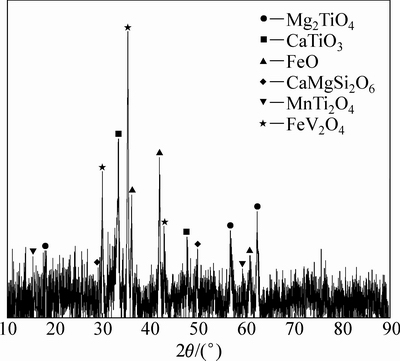
图1 含钛钢渣的XRD谱图
Fig. 1 XRD pattern of titanium slags

图2 含钛钢渣的SEM照片
Fig. 2 SEM image of titanium slags
实验试剂为NaOH,HCl和H2SO4,均为分析纯,由沈阳化工试剂厂生产;去离子水。
1.2 实验设备和检测仪器
实验设备为SX2-5-12型马弗炉、DHG-9070A型烘箱、ACS-1AL电子天平和SHZ-D(Ⅲ)循环水式真空泵。
通过JSM-6390A型扫描电镜检测钛渣和焙烧产物的形貌;通过Rigaku Ultima IV X线衍射仪检测钒渣和焙烧产物的物相,Cu靶Kα辐射(波长λ=1.540 6 nm),工作电压为40 kV,扫描范围2θ=5°~90°。
1.3 实验步骤
按碱矿比(NaOH与含钛钢渣的质量比)准确称取一定质量的NaOH和含钛钢渣,用研钵将其均匀混合,装入镍坩埚内,置于马弗炉中加热至设定温度并开始计时,焙烧过程中马弗炉半开门以保证样品在氧化性气氛中。反应结束后,待温度降到室温时,将镍坩埚放入预先盛有50 mL去离子水的烧杯中,之后再向坩埚中加入30 mL体积分数为5%的盐酸和30 mL体积分数为50%的硫酸。通过固液分离得到含钒溶液和浸出渣,将得到的渣保存,进行XRD和SEM结构表征分析,溶液中TiO2的质量分数采用硫酸高铁铵滴定法测定[13]。计算公式如下:
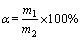 (1)
(1)
式中:α为TiO2的提取率;m1为滤液中TiO2的质量;m2为含钛钢渣中所含TiO2的质量。
2 结果与讨论
2.1 单因素实验
2.1.1 焙烧温度对TiO2提取率的影响
图3所示为在焙烧时间45 min、碱矿比6:1的条件下,焙烧温度与TiO2提取率的关系曲线。由图3可知:随焙烧温度的升高,TiO2提取率也逐渐增大。这是由于含钛钢渣在熔融NaOH体系中焙烧过程为吸热反应,焙烧温度升高,导致体系中NaOH黏度减小,反应阻力降低,固-固相反应转变为液-固相反应,促进反应进行。当焙烧温度超过450 ℃时,TiO2的提取率无明显变化,考虑能耗及生产成本,反应温度应控制在450 ℃为宜。

图3 焙烧温度对TiO2的提取率的影响
Fig. 3 Effect of roasting temperature on extraction rate of titanium dioxide
焙烧温度分别为350,400,450和500 ℃时反应过程渣的XRD谱如图4所示。由图4可知:当焙烧温度为350 ℃时,Mg2TiO4,MnTi2O4和CaTiO3衍射峰已消失,渣相主要为MgO,CaO,Fe2Ca2O4和MnO;当焙烧温度为450 ℃时,主要渣相为MgO,CaO,CaSiO3,Fe2Ca2O4和Fe2SiO4,含钛钢渣与体系中NaOH已完全反应,剩余硅酸盐相残留在渣中。
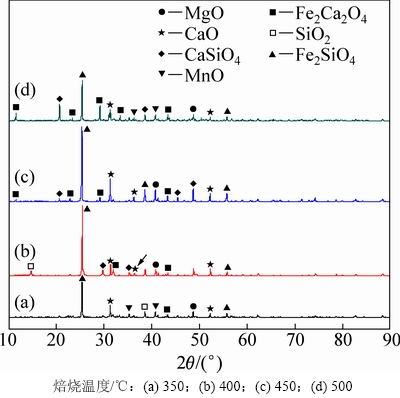
图4 不同焙烧温度时反应过程渣的XRD谱图
Fig. 4 XRD spectra of slag with different roasting temperatures
2.1.2 碱矿比对TiO2提取率的影响
图5所示为在焙烧温度450 ℃、焙烧时间45 min的条件下,碱矿比与TiO2提取率的关系曲线。由图5可知:当碱矿比3:1时,TiO2提取率为79.47%;当碱矿比增至6:1时,TiO2提取率达93.73%。当碱矿比继续增加时,TiO2提取率略有下降,说明碱量过多,容易造成体系中黏度增加、传质困难,不利于反应进行;同时在焙烧过程中易生成低熔点物质,导致TiO2提取率降低。
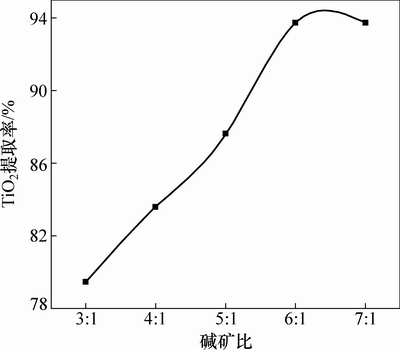
图5 碱矿比对TiO2提取率的影响
Fig. 5 Effect of alkali to ore ratio on extraction rate of TiO2
碱矿比分别为3:1,4:1,5:1,6:1时反应过程渣的XRD谱如图6所示。由图6可知:当碱矿比为3:1时,Mg2TiO4,MnTi2O4和CaTiO3在NaOH作用下生成Na2TiO3和相应的金属氧化物,FeV2O4在NaOH作用下生成钒酸盐和水;当碱矿比增至5:1时,随体系中NaOH量的增加,渣相中含钛化合物衍射峰逐渐减弱,反应趋于完全;当碱矿比达6:1时,含钛化合物衍射峰完全消失,由此也说明碱矿比为6:1时反应最充分。
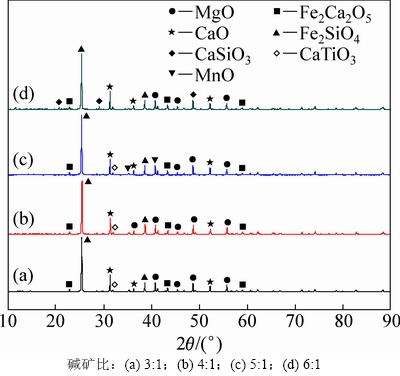
图6 不同碱矿比时反应过程渣的XRD谱图
Fig. 6 XRD spectra of slags at different alkali to ore ratios
2.1.3 焙烧时间对TiO2提取率的影响
图7所示为在焙烧温度450 ℃、碱矿比6:1的条件下,焙烧时间与TiO2提取率的关系曲线。由图7可知:当焙烧时间由15 min延长至45 min时,TiO2提取率由69.28%增至93.73%;当焙烧时间超过45 min时,TiO2提取率略有降低。这是由于焙烧时间过长,物料在高温下滞留的时间增加,更易导致烧结的发生,从而影响TiO2提取率。
焙烧时间分别为10,20,30和45 min时所得反应过程渣的XRD谱如图8所示。由图8可知:当焙烧时间为10 min时,主要赋存物相FeV2O4,Mg2TiO4和MnTi2O4衍射峰逐渐减弱,CaMgSi2O6则发生分解生成CaO,MgO和SiO2,渣相主要为CaTiO3,MgO,CaO,CaSiO3,Fe2Ca2O5和MnO,表明物料与NaOH已开始反应;当焙烧时间为20 min时,CaTiO3衍射峰消失,这是因为CaTiO3在NaOH作用下生成可溶性Na2TiO3和CaO,而CaO与体系中新生成的SiO2在焙烧温度下又生成CaSiO3,由于渣经过过滤、洗涤等步骤,反应生成的Na2TiO3易溶于水而进入液相,因此渣相中不存在Na2TiO3衍射峰。当焙烧时间为45 min时,渣相为MgO,CaO,Fe2Ca2O5,CaSiO3和Fe2SiO4,含钛化合物与NaOH已完全反应。通过XRD分析,可推测出含钛钢渣在熔融NaOH体系焙烧过程中相关反应如下:
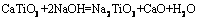 (2)
(2)
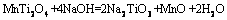 (3)
(3)
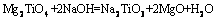 (4)
(4)
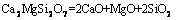 (5)
(5)
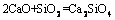 (6)
(6)

 (7)
(7)
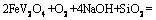
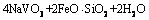 (8)
(8)
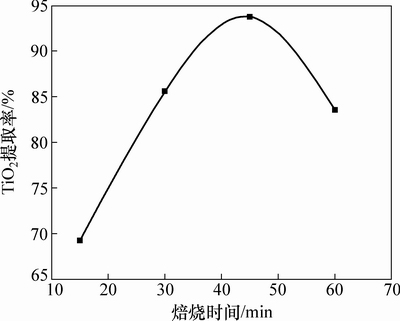
图7 焙烧时间对TiO2提取率的影响
Fig. 7 Effect of roasting time on extraction rate of TiO2
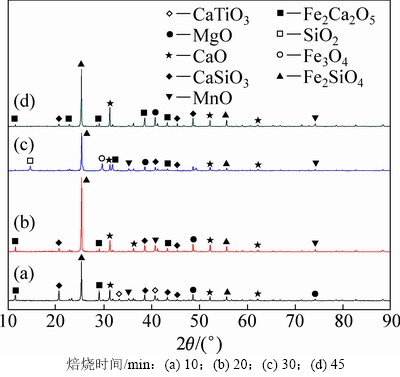
图8 不同焙烧时间时反应渣的XRD谱图
Fig. 8 XRD spectra of slags at different roasting time
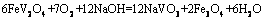 (9)
(9)
不同焙烧时间时反应渣的SEM照片如图9所示。由图9可知:随着焙烧时间的延长,含钛钢渣在熔融NaOH作用下表面逐步变得疏松。产生上述原因在于体系中存在较高浓度的OH-,对含钛钢渣中Mg2TiO4,MnTi2O4,CaTiO3可起到有效的分解作用,破坏其稳定的硅酸盐结构生成可溶性钛酸盐进入溶液,同时加快NaOH在焙烧体系中的扩散速度促进反应彻底进行。

图9 不同焙烧时间时反应渣的SEM照片
Fig. 9 SEM images of slag at different roasting time
2.2 正交实验
为探究各因素对含钛钢渣在熔融NaOH体系中焙烧过程的影响程度,在单因素实验的基础上设计正交实验。选择正交表L9(34)设计实验,以TiO2提取率为指标,选取焙烧温度、碱矿比、焙烧时间为3个正交因素,来确定含钛钢渣在熔融NaOH体系中焙烧过程的优化条件。各因素和水平见表2。
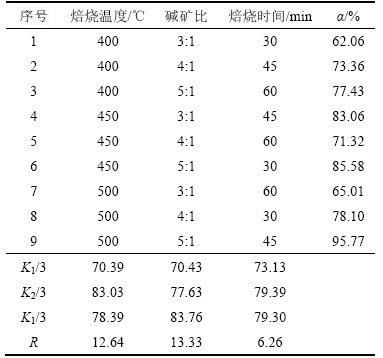
正交实验结果见表3,表中 K1,K2,K3分别表示一个因素各水平相应的实验结果之和,K1/3,K2/3,K3/3分别表示1个因素相应水平的平均值,并以此来判断优化反应条件;用R表示极差。通过极差法分析实验结果可知:1) 根据R可知各因素在选定范围内影响TiO2提取率的主次关系:碱矿比影响最为显著,其次是焙烧温度和焙烧时间;2) 优化反应条件如下:焙烧温度为450 ℃,碱矿比为6:1,焙烧时间为60 min。
表2 因素水平表
Table 2 Factors and levels
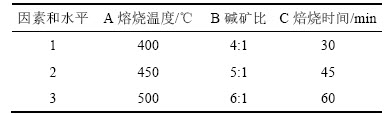
表3 正交实验结果
Table 3 Scheme of the orthogonal test
2.3 焙烧过程动力学
图10所示为在碱矿比6:1的条件下,不同焙烧温度、焙烧时间与TiO2提取率的关系曲线。含钛钢渣在熔融NaOH体系焙烧过程属于液-固反应,可选择收缩未反应核模型进行分析。其控制步骤可能是内扩散控制或化学反应控制。相应的动力学方程如式(10)和(11)所示:
 (10)
(10)
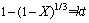 (11)
(11)
为进一步确定焙烧过程的控制步骤,将图10中的实验数据分别代入式(10)和(11)中进行拟合并作图,拟合结果如图11所示。由图11可知:1-2X/3-(1-X)2/3(图11(a))的线性相关性(R2=0.986 0)比1-(1-X)1/3(图11(b))的线性相关性(R2=0.903 4)更好,且与焙烧时间t呈较好的直线关系,说明含钛钢渣在碱熔NaOH体系焙烧过程可能是由内扩散控制。
根据Arrhenius方程:
 (12)
(12)
式中:A 为频率因子,min-1,E为活化能,J/mol,k为速率常数,min-1,R为理想气体常数,8.314 J/(K·mol)。
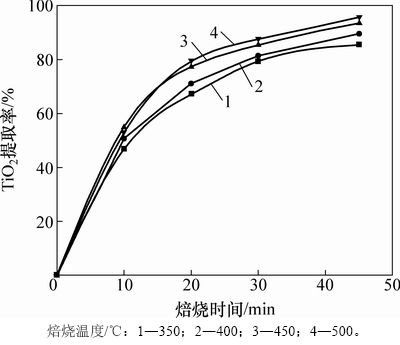
图10 不同焙烧温度下TiO2提取率随时间变化
Fig. 10 Extraction rate of TiO2 changed with time at different temperatures
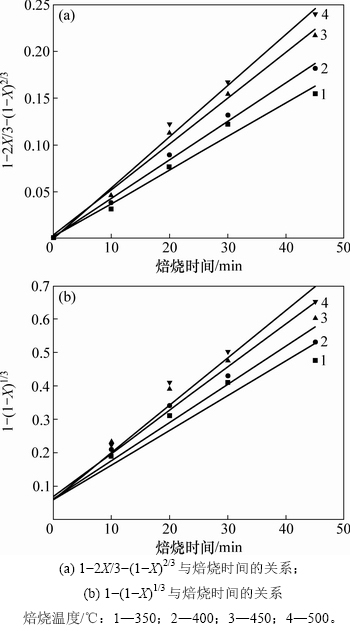
图11 不同焙烧温度下1-2X/3-(1-X)2/3和1-(1-X)1/3与焙烧时间的关系曲线
Fig. 11 Relationship between roasting time and 1-2X/3-(1-X)2/3 or 1-(1-X)1/3 at different temperatures
结合前面分析结果可知,含钛钢渣在碱熔NaOH体系焙烧过程是由内扩散控制。将图11(a)中的数据用线性回归处理可得不同焙烧温度时的反应速率常数k。以lnk对T-1作图(见图12)得到一条直线,计算得频率因子A=0.023 s-1,反应表观活化能E=10.77 kJ/mol,处于8~20 kJ/mol范围内(内扩散控制的反应活化能范围为8~20 kJ/mol)[14-15],由此可进一步判定在焙烧温度为350~500 ℃的范围内,含钛钢渣在熔融NaOH体系中焙烧的过程是由内扩散控制,其动力学方程可描述为
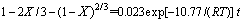 (13)
(13)
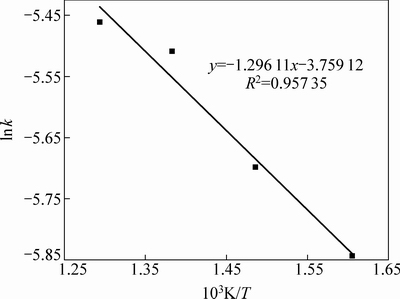
图12 lnk与T-1的关系
Fig. 12 Relationship between lnk and T-1
3 结论
1) 含钛钢渣在碱熔NaOH体系中焙烧过程优化反应条件为:焙烧温度450 ℃,碱矿比6:1,焙烧时间60 min。
2) 含钛钢渣在熔融NaOH体系焙烧过程动力学反应动力学符合未反应核收缩模型。结合TiO2在不同焙烧温度、焙烧时间时的提取率,发现在350~500 ℃的焙烧温度范围内,含钛钢渣在熔融NaOH体系中焙烧过程是由内扩散控制,反应表观活化能E为10.77 kJ/mol,其动力学方程可描述为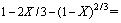
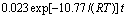 。
。
3) 含钛钢渣中主要物相Mg2TiO4,MnTi2O4和CaTiO3在熔融NaOH体系中焙烧过程中已完全分解,并获得含钛溶液,为后续钛的提取创造了有利条件。
参考文献:
[1] KADAM A N, DHABBE R S, KOKATE M R, et al. Preparation of N doped TiO2 via microwave-assisted method and its photocatalytic activity for degradation of malathion[J]. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy, 2014, 133(10): 669-676.
[2] MOHAMMADI S, HARVEY A, BOODHOO K V K. Synthesis of TiO2 nanoparticles in a spinning discreactor[J]. Chemical Engineering Journal, 2014, 258(15): 171-184.
[3] YU R, LIU Z, POURPOINT F, et al. Nanoparticulate TiO2 (B): an anode for lithium-ion batteries[J]. Angewandte Chemie International Edition, 2012, 51(9): 2164-2167.
[4] FENG X J, ZHU K, FRANK A J, et al. Rapid charge transport in dye-sensitized solar cells made from vertically aligned single-crystal rutile TiO2 nanowires[J]. Angewandte Chemie International Edition, 2012, 51(11): 2727-2730.
[5] ZHANG Y. Recovery of titanium from titanium bearing blast furnace slag by sulphate melting[J]. Canadian Metallurgical Quarterly, 2014, 53(4): 440-443.
[6] 王思佳, 张悦, 薛向欣, 等. 硫酸铵熔融反应法从含钛高炉渣中回收钛[J]. 化工学报, 2012, 63(3): 991-995.
WANG Sijia, ZHANG Yue, XUE Xiangxin, et al. Recovery of titanium from titanium-bearing blast furnace slag by ammonium sulfate melting method[J]. CIESC Journal, 2012, 63(3): 991-995.
[7] 霍东兴, 梁精龙, 李慧, 等. 含钛高炉渣提取钛的研究进展[J]. 热加工工艺, 2017, 46(3): 13-15.
HUO Dongxing, LIANG Jinglong, LI Hui, et al. Research progress on extracting titanium from blast furnace slag containing titanium[J]. Hot Working Technology, 2017, 46(3): 13-15.
[8] 李鑫, 于洪浩, 张侯芳, 等. 熔盐高效分解含钛高炉渣制备纳米二氧化钛[J]. 化工学报, 2015, 66(2): 827-833.
LI Xin, YU Honghao, ZHANG Houfang, et al. Preparation of nano TiO2 from Ti-bearing blast furnace slag by molten salt method[J]. CIESC Journal, 2015, 66(2): 827-833.
[9] HE Siqi, SUN Hongjuan, TAN Daoyong, et al. Recovery of titanium compounds from ti-enriched product of alkali melting Ti-bearing blast furnace slag by dilute sulfuric acid leaching[J]. Procedia Environmental Sciences, 2016, 31: 977-984.
[10] 熊瑶, 李春, 梁斌, 等. 盐酸浸出自然冷却含钛高炉渣[J]. 中国有色金属学报, 2008, 18(3): 557-563.
XIONG Yao, LI Chun, LIANG Bin, et al. Leaching behavior of air cooled Ti-bearing blast-furnace slag in hydrochloric acid[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(3): 557-563.
[11] 隋丽丽, 翟玉春. 硫酸氢铵焙烧高钛渣提取TiO2[J]. 中国有色金属学报, 2014, 24(3): 826-830.
SUI Lili, ZHAI Yuchun. Extraction of TiO2 from high titanium slag through roasting by ammonium bisulfate[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(3): 826-830.
[12] WU Zhang, LI Zhang, LI Yuhai, et al. An environmental procedure to extract titanium components and metallic iron from Ti-bearing blast furnace slag[J]. Green Processing and Synthesis, 2015, 4(4): 24-27.
[13] GRZMIL B U, GRELA D, KIC B. Hydrolysis of titanium sulphate compounds[J]. Chemical Papers, 2008, 62(1): 18-25.
[14] 赵昌明, 翟玉春, 刘岩, 等. 红土镍矿在NaOH亚熔盐体系中的预脱硅[J]. 中国有色金属学报, 2009, 19(5): 949-954.
ZHAO Changming, ZHAI Yuchun, LIU Yan, et al. Pre-desilication of laterite in NaOH sub-molten salt system[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(5): 949-954.
[15] 华一新. 冶金过程动力学导论[M]. 北京: 冶金工业出版社, 2004: 188-193.
HUA Yixin. Introduction to kinetics of metallurgy progress[M]. Beijing: Metallurgical Industry Press, 2004: 188-193.
(编辑 赵俊)
收稿日期:2017-12-14;修回日期:2018-04-06
基金项目(Foundation item):国家自然科学基金资助项目(51674141,51174111,51604147);辽宁省教育厅项目(2016TSPY02,2016TSPY03);辽宁省化学冶金工程重点实验室开放课题基金资助项目(USTLKFSY201502);辽宁省科技厅项目(201602394,201602401)(Projects(5167414, 51174111, 51604147) supported by the National Natural Science Foundation of China; Projects(2016TSPY02, 2016TSPY03) supported by the Educational Commission of Liaoning Province; Project(USTLKFSY201502) supported by the Key Laboratory of Chemical Metallurgy Engineering of Liaoning Province; Projects(201602394,201602401) supported by the Department of Science and Technology of Liaoning Province)
通信作者:赵昌明,博士,副教授,从事冶金物理化学研究;E-mail:lnzhaochangming@163.com