蒸发脱氨络合法制备的双氧水络合萃取分离钨钼前驱体料液
来源期刊:中国有色金属学报(英文版)2013年第4期
论文作者:关文娟 张贵清 高从堦
文章页码:1139 - 1146
关键词:钨;钼;萃取分离;前驱体料液;H2O2
Key words:tungsten; molybdenum; solvent extraction separation; precursor solution; H2O2
摘 要:采用一种新方法制备双氧水络合萃取分离钨钼的前驱体料液。高钼钨酸铵溶液经蒸发脱氨和络合转化步骤得到前驱体料液, 然后用三烷基氧膦(TRPO)和磷酸三丁酯(TBP)的混合萃取剂萃取分离钨和钼。结果表明:与传统的“直接调酸络合法”相比,“蒸发脱氨络合法”能减少90%以上的耗酸量;在络合转化过程中控制H2O2用量1.8~1.9倍、温度45~50 °C、初始pH值1.80~1.90、时间60 min和络合转化体积比100%,W、Mo的转化率高于95%,H2O2 的分解率低于15%;萃取过程中W的单级萃取率最低为2%,Mo的单级萃取率最高为82.6%,分离系数最高为76.7。
Abstract: An novel method on preparation of precursor solution for solvent separation of molybdenum (Mo) and tungsten (W) by hydrogen peroxide (H2O2)-complexation from the ammonium tungstate solution containing high Mo was studied. The precursor solution was obtained via evaporation deamination and H2O2-complex transformation processes. Then it was extracted with a mixture extractant of tri-alkyl phosphine oxide (TRPO) and tributyl phosphate (TBP) to separate Mo and W. The results indicated that the evaporation deamination complex method reduced the acid consumption by more than 90% in comparison with the traditional directly acid regulation complex method. The transformation rates of W and Mo were higher than 95% and the decomposition rate of H2O2 was less than 15% at a 1.8-1.9 times H2O2 dosage, 45-50 °C, initial pH of 1.80-1.90, and transformation volume ratio of 100% for 60 min in the H2O2-complexation transformation process. The minimum extraction rate of W was 2%, the maximum extraction rate of Mo was 82.6% and the highest separation coefficient was 76.7 in a single-stage extraction.
Trans. Nonferrous Met. Soc. China 23(2013) 1139-1146
Wen-juan GUAN1,2, Gui-qing ZHANG1,2, Cong-jie GAO1,2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Hunan Province for Metallurgy and Material Processing of Rare Metals, Central South University, Changsha 410083, China
Received 21 September 2012; accepted 22 November 2012
Abstract: An novel method on preparation of precursor solution for solvent separation of molybdenum (Mo) and tungsten (W) by hydrogen peroxide (H2O2)-complexation from the ammonium tungstate solution containing high Mo was studied. The precursor solution was obtained via evaporation deamination and H2O2-complex transformation processes. Then it was extracted with a mixture extractant of tri-alkyl phosphine oxide (TRPO) and tributyl phosphate (TBP) to separate Mo and W. The results indicated that the evaporation deamination complex method reduced the acid consumption by more than 90% in comparison with the traditional directly acid regulation complex method. The transformation rates of W and Mo were higher than 95% and the decomposition rate of H2O2 was less than 15% at a 1.8-1.9 times H2O2 dosage, 45-50 °C, initial pH of 1.80-1.90, and transformation volume ratio of 100% for 60 min in the H2O2-complexation transformation process. The minimum extraction rate of W was 2%, the maximum extraction rate of Mo was 82.6% and the highest separation coefficient was 76.7 in a single-stage extraction.
Key words: tungsten; molybdenum; solvent extraction separation; precursor solution; H2O2
1 Introduction
In recent years, with the continuous and heavy consumption of high-class tungsten resources [1], the content of Mo in the available resources is higher and higher [2,3]. Presently, the methods which have been widely used in industry are based on the property difference between thiomolybdate and tungstate [4-6]. These methods can meet the requirements for processing tungstate solution containing low Mo (m(Mo)/m(WO3)< 2%) but are not applicable to separate Mo from tungstate solution with high mass ratio of Mo to W because of too high cost [7]. It is of great significance to develop suitable process for the separation of Mo from tungstate solutions containing high content of Mo [8,9].
The method of solvent extraction separation of Mo and W by H2O2-complexation is based on the property difference between molybdenum peroxide and tungstate peroxide [10,11]. It provides obvious advantages including environmental friendliness and low cost, especially when the content of Mo is high. The precursor solution of this method is H2O2-complex solution by adding H2O2 into the mixed solution of W and Mo. However, W and Mo lead to a violent heterogeneous catalytic decomposition of H2O2 when pH>7 [12]. Thus, the alkaline tungstate solution containing Mo must be adjusted to be acidic (pH=2-4) before adding H2O2.
The sodium tungstate solution containing Mo can be modified to be acidic by directly adding inorganic acid [13-15], which is so-called “directly acid regulation complex method”. However, it is confronted with difficulties for the ammonium tungstate solution containing Mo by such a way. The first is the precipitation of a large amount of crystal which is hardly soluble in H2O2 solution at room temperature. The second is the high consumption of inorganic acid. For example, the ammonium tungstate solution containing high content of Mo was obtained from Luanchuan [16,17], in which the pH value is 9-10, the total NH4+ concentration is about 4 mol/L and there is 2-2.5 mol/L 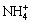 combined with
combined with 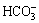 or
or 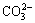 . Thus, the acid consumption of ammonium solution is much higher than that of sodium solution with a same pH regulation. The high acid consumption increases the cost of Mo/W separation and the large amount of inorganic salt formed during acid-base neutralization causes the environmental pollution.
. Thus, the acid consumption of ammonium solution is much higher than that of sodium solution with a same pH regulation. The high acid consumption increases the cost of Mo/W separation and the large amount of inorganic salt formed during acid-base neutralization causes the environmental pollution.
A novel method on preparation of the precursor solution from the ammonium tungstate solution was developed in this work. The precursor solution was obtained via processes of evaporation deamination and H2O2-complex transformation. In this method, the NH4HCO3 and (NH4)2CO3 in the ammonium solution were decomposed and removed in the form of NH3 and CO2 gases at high temperature, which lowered the pH of the solution and reduced the acid consumption. The removed NH3 and CO2 gases could be recovered by condensing. Thus, compared with the directly acid regulation complex method, the evaporation deamination complex method provides obvious advantages including low inorganic acid consumption, low cost and environmental friendliness. In this work, the behaviors of W, Mo and H2O2 in the evaporation deamination and H2O2-complex transformation processes under different conditions were investigated to explore the most appropriate experimental conditions. And the precursor solution was extracted with TRPO/TBP to determine whether it was qualified to separate Mo and W.
2 Experimental
2.1 Experimental flow
Figure 1 shows the experimental flow of preparation of the precursor solution from ammonium tungstate solution containing high Mo content by the evaporation deamination complex method.
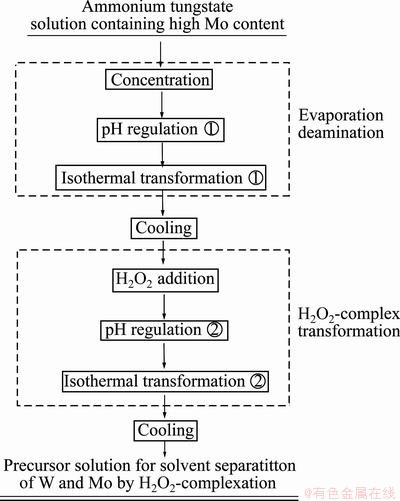
Fig. 1 Flowchart of preparation of precursor solution by evaporation deamination complex method for solvent separation of Mo and W by H2O2-complexation
2.2 Reagents
The ammonium tungstate solution contained 129 g/L WO3, 16.6 g/L Mo and its pH was 9.22. It was obtained from the process of directly solvent extraction of tungsten from autoclave-soda leaching liquor of scheelite and provided by a plant of the China Molybdenum Co., Ltd in Luanchuan, Henan Province, China. H2O2 was used as complex agent. Ammonium hydroxide (NH4OH) and the sulfuric acid (H2SO4) were used to adjust pH value of the solution. All reagents used were of analytical grade and the solutions were prepared in deionized water.
The organic extractants, tri-alkyl phosphine oxide (TRPO, industrial grade, purity>93%) and tributyl phosphate (TBP, analytical grade, purity>98.5%), were employed for extraction without purification. The commercial sulfonated kerosene was employed as diluent.
2.3 Experimental methods
The evaporation deamination tests were carried out by stirring and heating the ammonium tungstate solution samples of 500 mL in a beaker immersed in an electro thermostatic water bath for a specified time. The temperature was higher than 90 °C. The pH value of the concentrated solution was adjusted to 3-4 by adding 1:3 H2SO4 after evaporation concentration. Then a suitable amount of water was put into the beaker and the temperature continued to keep higher than 90 °C for about 0.5 h. After that, the crystallization slurry was removed from the water bath for cooling without solid-liquid separation.
The H2O2-complex transformation tests were carried out by mixing and heating a certain amount of H2O2 reagent and the crystallization slurry after cooling in a beaker immersed in an electro thermostatic water bath. The initial pH value was adjusted by adding 1:3 H2SO4 or 1:1 NH4OH and the transformation volume was adjusted by adding the pure water. The concentrations of WO3, Mo and H2O2 in the clarified complex solution were determined at regular intervals.
The extraction tests were carried out by mixing a certain volume organic phase and aqueous solution in a 250 mL-conical flask immersed in a water bath and constant temperature oscillator for a special time. After equilibration, the phases were allowed to separate in separating funnels and their volumes were measured. The concentrations of WO3 and Mo in aqueous were determined, and the extraction rates of WO3, Mo and the separation coefficient βMo/W were calculated.
The concentration of H2O2 was determined by potassium permanganate titration; the concentrations of WO3 and Mo in aqueous were determined by colorimetry using potassium thiocyanate and ammonium thiocyanate, respectively; the concentrations of WO3 and Mo in organic were calculated by subtraction method.
The concentrated volume ratio δ, the transformation volume ratio η and the H2O2 dosage Q were defined as
 (1)
(1)
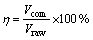 (2)
(2)
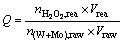 (3)
(3)
where V is the volume; n is the molar concentration; the subscripts “raw”, “con”, “com” and “rea” represent the ammonium tungstate solution, concentrated solution, H2O2-complex solution and H2O2 reagent, respectively.
High concentration of H2O2 resulted in a long stable standing time [16]. The stability of the H2O2-complex solution Q′ was defined as
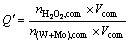 (4)
(4)
The transformation rates TM of M (WO3 or Mo) and the decomposition rate D of H2O2 were calculated as follows:
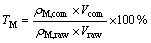 (5)
(5)
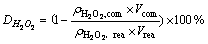 (6)
(6)
where ρ is mass concentration.
2.4 Experimental principle
The NH4HCO3 solution decomposed at temperature higher than 30 °C and the (NH4)2CO3 solution decomposed at temperature higher than 70 °C. Most of the NH4HCO3 and (NH4)2CO3 in the ammonium solution could be removed in the forms of NH3 and CO2 gases in the evaporation deamination process at a temperature higher than 90 °C, and the removed NH3 and CO2 could be recovered by condensing method. The reactions are as follows:
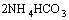 →
→ (7)
(7)
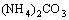 →
→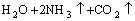 (8)
(8)
The pH value of the concentrated solution slowly decreased with the volatilization of ammonia. 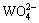 and
and 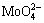 continuously polymerized to APT and secondary ammonium molybdenum (APM) at pH about 7. The reactions are as follows:
continuously polymerized to APT and secondary ammonium molybdenum (APM) at pH about 7. The reactions are as follows:
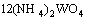 →
→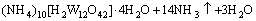 (9)
(9)
 →
→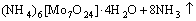 (10)
(10)
H2O2 was added into the crystallization slurry and it reacted with W or Mo to form tungstate peroxide or molybdenum peroxide. The reactions are as follows:
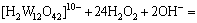
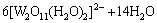 (11)
(11)
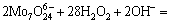
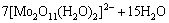 (12)
(12)
The complex reaction of APT with H2O2 progressed very slowly at room temperature. In order to increase the reaction rate, a certain temperature was kept during the H2O2-complex transformation process. The H2O2-complex solution after cooling was the precursor solution for solvent separation of Mo and W. The qualified precursor solution was clear, transparent and stable, and by extracting with TRPO/TBP, a good separation effect of W and Mo could be obtained.
3 Results and discussion
3.1 Evaporation deamination
Figure 2 shows the change law of the crystallization rates of W and Mo and the pH value of the concentrated solution in the evaporation deamination process at 90 °C. The results indicated that the pH value of the concentrated solution was decreased from 9.22 to 7.26 with the decrease of concentrated volume ratio δ from 100% to 17%. W began to crystallize when δ=80%. Although the solubility of APM in water was much greater than that of APT, a little Mo co-crystallized with W in the form of isomorphism-phase [18]. The crystallization rates of W increased from 5.50% to 84.41% with the decrease of concentrated volume ratio δ from 80% to 17%, while the crystallization rates of Mo increased from 3.99% to 32.01%.
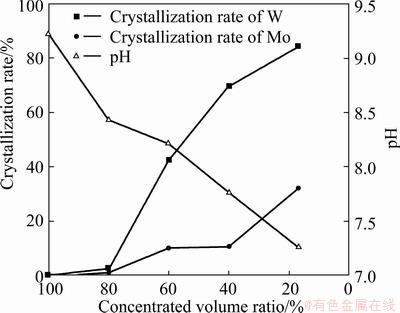
Fig. 2 Relationship between crystallization rates of W and Mo, solution pH and concentrated volume ratio
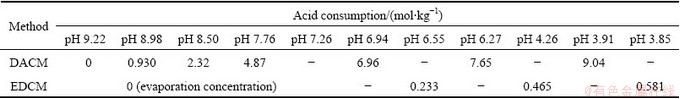
The relationship between the acid consumption and pH value was compared with that of the directly acid regulation complex method in Table 1. The results indicated that this method reduced acid consumption by more than 90% in comparison to the directly acid regulation complex method because the solution pH value had decreased from 9.22 to 7.26 in the evaporation concentration process and most of the NH4HCO3 and (NH4)2CO3 had been removed.
3.2 H2O2-complex transformation
3.2.1 Effects of H2O2 dosage
The effects of H2O2 dosage on the H2O2-complex transformation process was investigated in the range of 1.62-2.0 times [10]. The result is shown in Fig. 3.
It can be seen from Figs. 3(a) and (b) that the initial transformation rate of W was 14%-18%, but that of Mo was 72%-82%. The reason was that the crystallization rate of W was much higher than that of Mo in the evaporation deamination process. The transformation rates of W and Mo increased with the increase of transformation time at a constant H2O2 dosage or increased with the increase of H2O2 dosage at a constant transformation time. This result indicated that high dosage of H2O2 resulted in fast complex reaction of W and Mo with H2O2. Figure 3(c) shows that the decomposition rate of H2O2 increased with the increase of transformation time or decreased with the increase of H2O2 dosage. Turbidity was seen in the H2O2-complex solution at a H2O2 dosage of Q=1.6 times for 55 min, while the decomposition rate of H2O2 was 24.86% and Q′ in the H2O2-complex solution was 1.24. In order to ensure the stability of the complex solution, the Q′ value should be higher than 1.4.

Fig. 3 Effects of H2O2 dosage on H2O2-complex transformation process at initial pH 1.80, temperature 50 °C and transformation volume ratio 100%
Table 1 Relationships between acid consumption and pH value of evaporation deamination complex method (EDCM) and directly acid regulation complex method (DACM)
A H2O2 dosage of Q=1.8-2.0 times was thought to be optimum. Under the experimental condition, the transformation rates of W and Mo were higher than 95% and the decomposition rate of H2O2 was lower than 20% for a transformation time of 60 min, while the Q′ values in the H2O2-complex solutions were higher than 1.5 and the solutions were clear and transparent during the experiment.
3.2.2 Effects of transformation temperature and time
Figure 4 shows the effects of transformation temperature and time on the H2O2-complex transformation process. It can be seen from Fig. 4 that the transformation rates of W and Mo and the decomposition rate of H2O2 increased with the increase of transformation time at a constant temperature or increased with the increase of transformation temperature at a constant time. The result indicated that high temperature resulted in fast complex reaction of W and Mo with H2O2 and fast decomposition of H2O2 or H2O2-complex.
The transformation rates of W and Mo changed a little with the transformation time from 60 min to 120 min at 40 °C, 40-90 min at 50 °C and 35-55 min at 60 °C. To ensure high transformation rates of W and Mo, low decomposition rate of H2O2 and good stability of the H2O2-complex solution, a temperature range of 45-50°C and time of 60 min were selected.
3.2.3 Effects of initial pH value of H2O2-complex solution
The initial pH value of the H2O2-complex solution before pH regulation was 1.6-2.1 at a H2O2 dosage of Q=1.8-2.0 times. It can be seen from Eqs. (11) and (12) that OH- was consumed during the complex reactions of W and Mo with H2O2. Thus, the pH value decreased in the process of H2O2-complex transformation.
Figure 5 shows the effects of initial pH value on the H2O2-complex transformation process. The result indicated that the initial pH value of H2O2-complex solution had a little influence on the transformation rates of W and Mo. The decomposition rate of H2O2 decreased with the increase of initial pH value. An initial pH value of 1.8-1.9 was considered to be optimum.
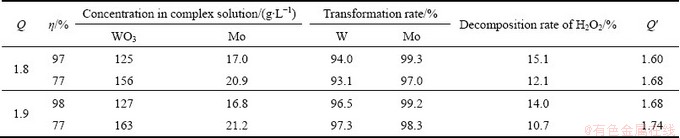
3.2.4 Effects of transformation volume ratio
Table 2 shows the effects of the transformation volume ratio η on the H2O2-complex transformation process. The result indicated that the transformation volume ratio of η=77%-98% had little influence on the transformation rates of W and Mo, and the stabilities of all of the H2O2-complex solutions obtained at different transformation volume ratios were good. However, low transformation volume ratio η resulted in low decomposition rate of H2O2 and high concentrations of W and Mo in the H2O2-complex solution. Thus, the transformation volume ratio η could be selected according to the practical needs of concentrations of W and Mo in the extraction process.
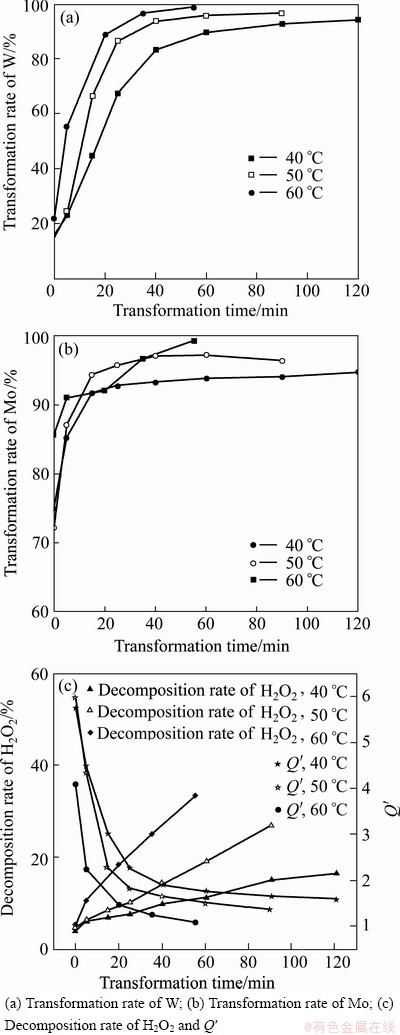
Fig. 4 Effects of transformation temperature and time on H2O2-comple transformation process at H2O2 dosage of Q=1.8 times, initial pH 1.80 and transformation volume ratio η=100%
Under the optimum conditions of H2O2 dosage Q=1.8-1.9 times, temperature of 45-50 °C, time of 60 min, initial pH value of 1.80-1.90 and transformation volume ratio η=100%, the transformation rates of W and Mo were higher than 95%, the decomposition rate of H2O2 was lower than 15% and the Q′ value in the H2O2-complex solution was higher than 1.6.
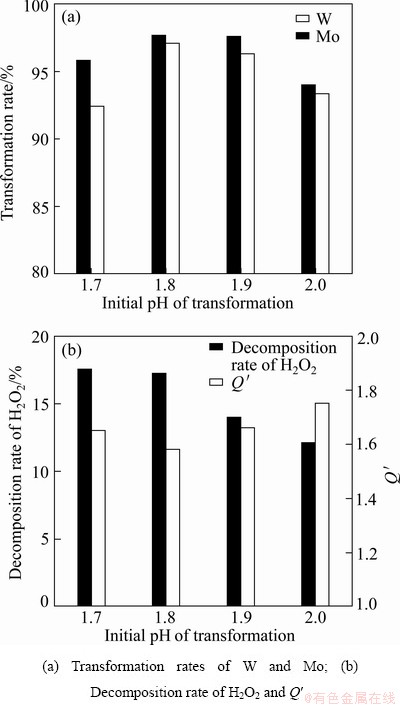
Fig. 5 Effects of initial pH value of H2O2-complex solution on H2O2-complex transformation process at H2O2 dosage of Q=1.9, temperature 45 °C and transformation volume ratio η=100% for 60 min
There was large crystals of W and Mo not dissolved in the H2O2-complex transformation process because large crystals resulted in slow dissolution. These crystals were returned to the evaporation deamination process after solid-liquid separation, and they were re-dissolved in the ammonium tungstate solution at the beginning of the evaporation concentration. Thus the practical utilization ratios of W and Mo in the evaporation deamination complex method were close to 100%.
3.3 Extraction
The precursor solution prepared by the evaporation deamination complex method contained 161.3 g/L WO3, 20.29 g/L Mo and pH was 1.75. The Q′ value in the precursor solution was 1.70. It was extracted with TRPO/TBP to separate W and Mo. The result is shown in Fig. 6.

Fig. 6 Result of solvent separation of W and Mo by H2O2- complexation using aqueous solution containing 127-129 g/L WO3, 15.9-16.1 g/L Mo with 2% (v/v) TRPO and 80% (v/v) TBP at oil to water (O/A) phase ratio of 2:1, temperature 20 °C and contact time 5 min
It can be seen from Fig. 6 that the extraction rates of W and Mo decreased with the increase of initial pH value of aqueous solution. The minimum extraction rate of W was 2%, the maximum extraction rate of Mo was 82.6% and the highest separation coefficient was 76.7 in a single-stage extraction. The phase separation property in extraction was good. The aqueous solution and raffinate were clear, transparent and stable. The results demonstrated that the precursor solution prepared by the evaporation deamination complex method could meet the requirements for separation of W and Mo by H2O2- complexation [10,11].
Table 2 Effects of transformation volume ratio η on H2O2-complex transformation process at initial pH of 1.90 and temperature of 45 °C for 60 min
4 Conclusions
1) The qualified precursor solution for solvent separation of Mo and W by H2O2-complexation was prepared by the evaporation deamination complex method. This method has reduced acid consumption by more than 90% in comparison with the traditional directly acid regulation complex method. The high transformation rates of W and Mo and low decomposition rate of H2O2 have been ensured. The precursor solution was clear, transparent and stable, and by extracting with TRPO/TBP, a good separation effect of W and Mo has been obtained.
2) Most of the NH4HCO3 and (NH4)2CO3 in the ammonium tungstate solution containing high content of Mo have been removed in the forms of NH3 and CO2 gases in the evaporation deamination process. pH value of the solution decreased from 9.22 to 7.26 with the decrease of concentrated volume ratio δ from 100% to 17%, and then the crystallization rates of W and Mo were 84.41% and 32.01%, respectively.
3) Under optimum conditions of H2O2 dosage of Q=1.8-1.9 times, temperature of 45-50 °C, time of 60 min, initial pH of 1.80-1.90 and transformation volume ratio of η=100%, the transformation rates of W and Mo were higher than 95%, the decomposition rate of H2O2 was lower than 15% and the Q′ value in the H2O2-complex solution was higher than 1.6. The practical utilization ratios of W and Mo in this method were close to 100%.
4) Separation of W and Mo from the precursor solution prepared by the evaporation deamination complex method has been carried out by using a mixture extractant of 2% (v/v) TRPO and 80% (v/v) TBP. The minimum extraction rate of W was 2%, the maximum extraction rate of Mo was 82.6% and the highest separation coefficient βMo/W was 76.7 in a single-stage extraction at an oil to water ratio of 2:1, temperature of 20 °C and contact time of 5 min.
Acknowledgement
The authors want to thank Professor Qi-xiu ZHANG (School of Metallurgical Science and Engineering, Central South University) for valuable discussions concerning this work. The authors also express sincere thanks to the China Molybdenum Co., Ltd. for financial support.
References
[1] KONG Zhao-qin. On the development of China’s tungsten industry in the past 60 years [J]. Chinese Tungsten Industry, 2009, 24(5): 1-10. (in Chinese)
[2]  Ying, LI Hong-gui. Utilize tungsten concentrates of high molybdenum rationally [J]. Chinese Tungsten Industry, 2005, 20(5): 15-21. (in Chinese)
Ying, LI Hong-gui. Utilize tungsten concentrates of high molybdenum rationally [J]. Chinese Tungsten Industry, 2005, 20(5): 15-21. (in Chinese)
[3] LI Qing-gang, ZHANG Qi-xiu, LI Zan-en, TIAN Ji-ying, XIAO Lian-sheng. On the recovery of tungsten and molybdenum out of acid leaching solution by ion-exchange [J]. Chinese Tungsten Industry, 2009, 24(5): 56-59, 129. (in Chinese)
[4] HUANG Wei-zhuang, GONG Bo-fan, ZHANG Qi-xiu. Separation of tungsten and molybdenum from thiomolybdate salts by solvent extraction [J]. The Chinese Journal of Nonferrous Metals, 1995, 5(1): 45-47. (in Chinese)
[5] XIAO Lian-sheng, ZHANG Qi-xiu, GONG Bo-fan, HUAN Shao-ying. The industrial application of removing molybdenum technology in packed moving bed by fluid bed ion-exchange [J]. China Tungsten Industry, 2001, 16(2): 26-29. (in Chinese)
[6] LI Hong-gui, HUO Guang-sheng, SUN Pei-mei, ZHAO Zhong-wei, LI Yun-jiao, SU Peng-tuan, LIU Mao-sheng. Developing new reagent for selectively precipitation of molybdenum from tungsten solution [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(1): 184-187.
[7] LI Hong-gui. Production of high purity APT from scheelite and complex tungsten raw material with high Mo content [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(2): 366-369.
[8] ZHAO Zhong-wei, CAO Cai-fang, CHEN Xing-yu. Separation of macro amounts of tungsten and molybdenum by precipitation with ferrous salt [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(12): 2758-2763.
[9] ZHAO Zhong-wei, CAO Cai-fang, CHEN Xing-yu, HUO Guang-sheng. Separation of macro amounts of tungsten and molybdenum by selective precipitation [J]. Hydrometallurgy, 2011, 108: 229-232.
[10] GUAN Wen-juan, ZHANG Gui-qing, GAO Cong-jie. Solvent extraction separation of molybdenum and tungsten from ammonium solution by H2O2-complexation [J]. Hydrometallurgy, 2012, 127-128: 84-90.
[11] OU Hui, ZHANG Gui-qing, GUAN Wen-juan, XIAO Lian-sheng. On separating molybdenum from tungsten by solvent extraction with mixed extractant using H2O2 as complex agent [J]. Chinese Tungsten Industry, 2011, 26(3): 34-36. (in Chinese)
[12]  Xi-lun, Inorganic peroxide complex chemistry [M]. Beijing: Science Press, 1987: 422. (in Chinese)
Xi-lun, Inorganic peroxide complex chemistry [M]. Beijing: Science Press, 1987: 422. (in Chinese)
[13] ZELIKMAN A N, VOLDMAN G M, RUMYANTSEV V K, VIKTOR K, ZIBEROV G N, KAGERMANIAN V S. Process for separation of tungsten and molybdenum by extraction: USA, 3969478 [P]. 1976-07-13.
[14] OZENSOY E, BURKIN A R. Separation of tungsten and molybdenum by solvent extraction: USA, 4275039 [P]. 1981-06-23.
[15] LI Wei-xuan, ZHANG Li-yi. Separation of tungsten and molybdenum from acid solution by selective extraction [J]. Chinese Journal of Rare Metals, 1990, 14(4): 251-255. (in Chinese)
[16] GUAN Wen-juan, ZHANG Gui-qing. Extraction of tungsten from simulated autoclave-soda leaching liquor of scheelite with quaternary ammonium salt [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(7): 1756-1762. (in Chinese)
[17] ZHANG Gui-qing, GUAN Wen-juan, ZHANG Qi-xiu, XIAO Lian-sheng, LI Qing-gang, CAO Zuo-ying. Continuous-running experiment for direct solvent extraction of tungsten from autoclave–soda leaching liquor of scheeite [J]. China Tungsten Industry, 2009, 24(5): 49-52. (in Chinese)
[18] LI Hong-gui, YANG Jian-gao, LI Kun. Tungsten metallurgy [M]. Changsha: Central South University Press, 2010: 236-237. (in Chinese).
关文娟1,2,张贵清1,2,高从堦1,2
1. 中南大学 冶金科学与工程学院,长沙 410083;
2. 中南大学 稀有金属冶金与材料制备湖南省重点试验室,长沙 410083
摘 要:采用一种新方法制备双氧水络合萃取分离钨钼的前驱体料液。高钼钨酸铵溶液经蒸发脱氨和络合转化步骤得到前驱体料液, 然后用三烷基氧膦(TRPO)和磷酸三丁酯(TBP)的混合萃取剂萃取分离钨和钼。结果表明:与传统的“直接调酸络合法”相比,“蒸发脱氨络合法”能减少90%以上的耗酸量;在络合转化过程中控制H2O2用量1.8~1.9倍、温度45~50 °C、初始pH值1.80~1.90、时间60 min和络合转化体积比100%,W、Mo的转化率高于95%,H2O2 的分解率低于15%;萃取过程中W的单级萃取率最低为2%,Mo的单级萃取率最高为82.6%,分离系数最高为76.7。
关键词:钨;钼;萃取分离;前驱体料液;H2O2
(Edited by Hua YANG)
Foundation item: Project (2010ZX07212-008) supported by the Major Science and Technology Program for Water Pollution Control and Treatment
Corresponding author: Gui-qing ZHANG; Tel: +86-731-88830472; E-mail: gq_zhang@163.com
DOI: 10.1016/S1003-6326(13)62576-5

