
Effect of silicate pretreatment, post-sealing and additives on corrosion resistance of phosphated galvanized steel
XU Yu-ye(徐玉野)1, LIN Bi-lan(林碧兰)2
1. College of Civil Engineering, Huaqiao University, Quanzhou 362021, China;
2. College of Materials Science and Engineering, South China University of Technology,
Guangzhou 510640, China
Received 15 July 2007; accepted 10 September 2007
Abstract: Sodium silicate (water glass) pretreatment before phosphating, silicate post-sealing after phosphating and adding silicate to a traditional phosphating solution were respectively carried out to obtain the improved phosphate coatings with high corrosion resistance and coverage on hot-dip galvanized(HDG) steel. The corrosion resistance, morphology and chemical composition of the coatings were investigated using neutral salt spray(NSS) tests, scanning electron microscopy(SEM) and energy dispersive spectroscopy(EDS). The results show that pretreatment HDG steel with silicate solutions, phosphate coatings with finer crystals and higher coverage are formed and the corrosion resistance is enhanced. Adding silicate to a traditional phosphating solution, the surface morphology of the coatings is nearly unchanged. The corrosion resistance of the coatings is mainly dependent on phosphating time. Phosphating for a longer time (such as 5 min), the corrosion resistance, increasing with concentration of silicate, is improved significantly. Post-sealing the phosphated HDG steel with silicate solutions, the pores among the zinc phosphate crystals are sealed with the films containing Si, P, O and Zn and the continuous composite coatings are formed. The corrosion resistance of the composite coatings, related to the pH value, contents of hydrated gel of silica and  and post-sealing time, is increased markedly. The improved coatings with optimal corrosion resistance are obtained for phosphating 5 min and post-sealing with 5 g/L silicate solution for 10 min.
and post-sealing time, is increased markedly. The improved coatings with optimal corrosion resistance are obtained for phosphating 5 min and post-sealing with 5 g/L silicate solution for 10 min.
Key words: galvanized steel; phosphate coatings; silicate; corrosion resistance
1 Introduction
Phosphating has been widely applied in many industries for corrosion protection, primer for painting, wear reduction, metal-forming lubricants and electrical insulation[1]. Corrosion resistance is important to evaluate the performance of phosphate coatings, which determines the applications of the coatings. Recent efforts to enhance the corrosion resistance of phosphate coatings have mainly been focused on the pretreatment methods before phosphating and the process technologies for phosphating[2-4]. The primary object is to increase the coverage of the coatings.
However, phosphate coatings are crystalline and porous. The pores can not be eliminated thoroughly by one-step phosphating, requiring a post-treatment after phosphating. A mixture of hexavalent and trivalent chromate, or dilute chromic acid solutions have been used traditionally for the post-treatment[5]. The serious environmental difficulties connected with chromate promote the development of chrome-free alternatives [6-7], but few chrome-free approaches provide the same corrosion resistance as that of chromate.
Silicate is colloidal and is an effective corrosion inhibitor for iron and steel in cooling water systems[8]. Aramaki[8] has found that sodium silicate Na2Si2O5 is remarkably effective on zinc corrosion in 0.5 mol/L NaCl, and a layer composed of Zn(OH)2 (about 10 nm) and a small quantity of 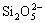 compounds, which suppresses the cathodic oxygen reduction and the anodic zinc dis- solution respectively, is formed. The effects of silicate have been investigated as a sealing step after cerium treatment into thick aluminum oxide layer[9].
compounds, which suppresses the cathodic oxygen reduction and the anodic zinc dis- solution respectively, is formed. The effects of silicate have been investigated as a sealing step after cerium treatment into thick aluminum oxide layer[9].
They have found that silicate fills the pores of cellular oxide structure and the silicate layer acts as a barrier to oxygen diffusion to the metal surface. Shi et al[10] have pretreated AZ31 magnesium alloy in a bath containing 20 g/L KMnO4, 60 g/L Na2HPO4·7H2O and 25 mL/L HNO3 and then sealed in a 10 g/L sodium silicate bath at 85 ℃ for 10 min, and have found that the corrosion resistance is greatly improved. Hamdy et al[9,11-12] have studied the effect of silicate post-sealing on the corrosion behaviors of aluminum alloy pretreated with cerate or permanganate solutions. They conclude that silica plays an important role in the pitting repair process.
So far, the effect of silicate pretreatment, post-sealing and additives on the corrosion resistance of phosphate coatings on HDG steel has not been documented yet. In this work, the corrosion resistance, morphology and chemical composition of the silicate improved phosphate coatings were studied and compared with the traditional single phosphate coatings.
2 Experimental
Cold rolled sheets Q235 of 50 mm×40 mm×2 mm were degreased, pickled, fluxed, dried and dipped in a zinc bath at 450 ℃ for 1 min, then withdrawn slowly and quenched in water immediately. The thickness of the galvanized layer was about 50 μm.
In the previous study[13-14], the process technologies for the traditional phosphating are listed in Table 1. The coverage of the traditional single phosphate coatings increases with phosphating time and phosphate coatings with a better corrosion resistance are obtained after 5 min phosphating. In this work, the process technologies for the traditional phosphating are also chosen listed in Table 1, and those for the sodium silicate treatment (including pretreatment and post-sealing treatment) are also listed in Table 1. Sodium silicate is a hydrated solution containing 19% Na2O and 38% SiO2. The solutions were prepared from reagent grade chemicals and de-ionized water. The addition content of silicate additives to the traditional phosphating solution was 0.1-10 g/L.
NSS tests were conducted using 5% NaCl solution with pH 6.5-7.0 at (35±2) ℃. The samples were placed
Table 1 Process technologies for phosphating and sodium silicate treatment

perpendicularly with an angle of 30?. In a spray cycle, the samples were continuously sprayed for 8 h and then kept in NSS chamber for 16 h. The corroded area of zinc was recorded after each spray cycle.
The surface morphology of the coated samples was observed by SEM (PHILIPS; Model: XL-30-FEG), and the chemical composition of the coatings was analyzed by EDS (EDAX; Model: DX-4).
3 Results and discussion
3.1 Effect of silicate pretreatment on corrosion resistance and morphology of phosphate coatings
Fig.1 shows the NSS results of the 5 min phosphated HDG samples pretreated with variable concentration of sodium silicate solutions for 10 min. Pretreatment HDG steel with silicate solutions and subsequent immersion in the traditional phosphating solution, the corrosion resistance of the coatings, unrelated with the concentration of silicate solutions, is enhanced greatly. Immersion HDG steel in silicate solutions, a protective films composed of a hydrated gel of silica, zinc silicate and (or) zinc hydroxide may be formed[8]. Neighboring these compounds, the chemical activity is higher, facilitating the precipitation of the zinc phosphate crystals. Therefore, the crystal nuclei of zinc phosphate are increased, and the coatings with finer crystals and higher coverage are formed, as shown in Fig.2. This may be similar to the activation mechanism of the sodium titanium phosphate colloid for the formation of phosphate coatings[3].

Fig.1 NSS results of 5 min phosphated HDG samples pretreated with variable concentration of silicate solutions for 10 min
3.2 Effect of silicate additives on corrosion resistance and morphology of phosphate coatings

Fig.2 SEM micrographs of 5 min phosphated HDG samples: (a) Without pretreatment; (b) Pretreated with 5 g/L silicate solution for 10 min
Fig.3 shows the NSS results of HDG steel phosphated in the phosphating solution containing variable addition content of silicate additives for different times. Adding silicate to the traditional phosphating solution, the corrosion resistance of the improved phosphate coatings is mainly dependent on phosphating time. This is similar to that of the traditional single phosphate coatings[13-14]. Phosphating for a shorter time (such as 1 min), the corrosion resistance of phosphate coatings is enhanced with a moderate addition content of silicate additives on the whole (such as 1-5 g/L), but it is decreased with a less or a higher addition content of silicate additives on the whole (such as 0.1 g/L or 10 g/L), as shown in Fig.3(a). For phosphating time longer than 5 min, silicate additives greatly improve the corrosion resistance of phosphate coatings no matter how much the addition content of silicate additives is (Fig.3(b)). When the phosphating time is increased to 10 min, the corrosion resistance of the coatings is also greatly increased with the increase in addition content of silicate additives on the whole (Fig.3(c)).
Silica is metastable when the pH value of the solution is 2-3. Colloidal silicate may be occluded in the skeleton of the zinc phosphate crystals. With the increasing of phosphating time, the coverage of phosphate coatings is increased, the content of silica inclusions is thereby increased, resulting in the enhance-
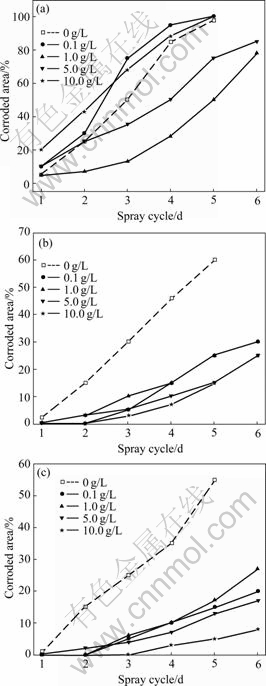
Fig.3 NSS results of HDG steel phosphated in phosphating solution containing variable addition content of silicate additives for different times: (a) 1 min; (b) 5 min; (c) 10 min
ment of the corrosion resistance of phosphate coatings. Meanwhile, for a higher addition content of silicate additives, the amount of silica inclusions is accordingly higher, leading to the increase in corrosion resistance. However, compared with the effect of the addition content of silicate additives on the corrosion resistance of phosphate coatings, phosphating time is fairly more important to increase the corrosion resistance of phosphate coatings.
SEM observations show that adding silicate to the traditional phosphating solution, the microstructure and size of the zinc phosphate crystals are nearly unchanged as shown in Fig.4. Zinc phosphate crystals are still needle-like and the size of the zinc phosphate crystals is more than 50 μm. Table 2 shows the chemical compositions of different micro-sites. In Table 2, the element of Si is detected on the surface of both the needle-like zinc phosphate crystals and the pores when the silicate additives is added to the traditional phosphating solution, whereas Zn and a small amount of O are detected on the pores of the traditional single phosphate coatings. This indicates that during immersion HDG steel in the phosphating solution containing silicate additives, colloidal silica maybe also participate in the formation of the coatings and the coverage of the coatings is thus increased.

Fig.4 SEM micrograph of HDG steel phosphated in phosphating solution containing 10 g/L silicate additives for 10 min
Table 2 Chemical compositions of different micro-sites (mass fraction, %)

3.3 Effect of silicate post-sealing on corrosion resistance and morphology of phosphate coatings
NSS results show that the corrosion resistance of the phosphated HDG samples is greatly enhanced by the sodium silicate post-sealing treatment. Fig.5 shows the NSS results of the 5 min phosphated HDG samples post-sealed with variable concentration of silicate
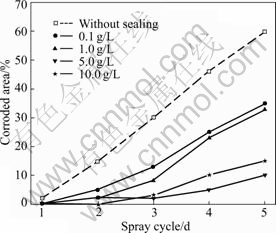
Fig.5 NSS results of 5 min phosphated samples post-sealed with variable concentration of silicate solutions for 1 min
solutions for 1 min. The corrosion resistance of the coatings increases with the increase in concentration of silicate in the range between 0.1 g/L and 5 g/L but appears to decrease slightly for a higher concentration on the whole.
Fig.6 shows the NSS results of the 5 min phosphated HDG samples post-sealed with 5 g/L sodium silicate solution for different times. In the case of 5 g/L sodium silicate, the corrosion resistance of the coatings increases with the increase in post-sealing time up to 10 min but decreases during a longer post-sealing treatment. When the post-sealing time is 10 min, the composite coatings with optimal corrosion resistance are obtained and no corrosion is observed in six spray cycles, whereas the corrosion appears on the phosphated HDG sample without the silicate post-treatment in only one spray cycle.
The pH value of a silicate solution more than 6 is required for the formation of colloidal silica[15]. The pH values of the solutions with 0.1, 1, 5 and 10 g/L silicate were 8.4, 9.6, 10.4 and 10.8, respectively. Probably, colloidal silica is formed in these solutions. Parashar et al[16] have found that the chemical resistance properties, including corrosion resistance, of water-borne inorganic silicate films depend on the ratio of silica to alkali metal oxide. Post-sealing the phosphated HDG samples with silicate solutions, a hydrated gel of silica may be formed in the pores among the zinc phosphate crystals, resulting in the increase in corrosion resistance of phosphate coatings.
With the increasing of the concentration of silicate solutions, the amount of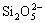 compounds and colloidal silica are thereby increased. The more protective films composed of zinc silicate and hydrated gel of silica are formed. Therefore, the corrosion resistance of phosphate coatings is enhanced.
compounds and colloidal silica are thereby increased. The more protective films composed of zinc silicate and hydrated gel of silica are formed. Therefore, the corrosion resistance of phosphate coatings is enhanced.
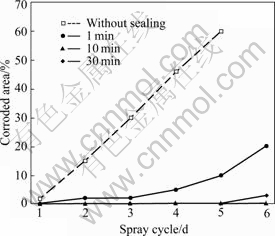
Fig.6 NSS results of 5 min phosphated HDG samples post-sealed with 5 g/L silicate solution for different times
According to the E—pH diagram for Zn-H2O system[17], if pH<8.46, 8.46-10.62 and >10.62, the existence forms of zinc are Zn2+, Zn(OH)2 and 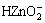 respectively, where the compounds of Zn2+ and
respectively, where the compounds of Zn2+ and 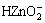 are soluble but Zn(OH)2 compound is insoluble and protective. For a less or a higher concentration of silicate solutions (such as 0.1 g/L or 10 g/L), the protective films to improve the corrosion resistance of phosphate coatings may be composed of zinc silicate and hydrated gel of silica. For a moderate concentration of silicate solutions (such as 1-5 g/L), Zn(OH)2 compound may also be formed, resulting in a greater enhancement of the corrosion resistance of the coatings.
are soluble but Zn(OH)2 compound is insoluble and protective. For a less or a higher concentration of silicate solutions (such as 0.1 g/L or 10 g/L), the protective films to improve the corrosion resistance of phosphate coatings may be composed of zinc silicate and hydrated gel of silica. For a moderate concentration of silicate solutions (such as 1-5 g/L), Zn(OH)2 compound may also be formed, resulting in a greater enhancement of the corrosion resistance of the coatings.
SEM results show that after post-sealing the phosphated HDG samples with silicate solutions, the surface morphology appears to be unchanged, as shown in Fig.7. EDS data in Table 2 shows that compared with the chemical compositions on the pores of the traditional single phosphate coatings, the elements of Si, P, Zn and a high content of O are detected on the pores among the zinc phosphate crystals after the silicate post-sealing treatment. This indicates that some new compounds are

Fig.7 SEM micrograph of 5 min phosphated HDG sample post-sealed with 5 g/L silicate solution for 10 min
coated on the pores and the continuous composite coatings are formed on the surface of HDG steel.
NSS results also show that the corrosion resistance of the single silicate films appears to be poor and the corroded area of zinc is more than 90% after 8 h salt- spray. This may be due to that silicate films are thin. In addition, the corrosion resistance of the silicate improved phosphate coatings is increased in the following order: silicate post-sealing treatment>silicate pretreatment>silicate additives to the traditional phosphating solution.
4 Conclusions
1) Pretreatment HDG steel with silicate solutions, the improved coatings with finer crystals and higher coverage are formed and the corrosion resistance of the coatings is greatly enhanced.
2) Adding silicate to the phosphating solution, the morphology of the improved coatings appears to be unchanged but the element of Si is detected on the whole surface; the corrosion resistance of the coatings is mainly dependent on phosphating time.
3) Post-sealing the phosphated HDG steel with silicate solutions, the pores among the zinc phosphate crystals are sealed with the films containing Si, P, O and Zn, and the continuous composite coatings are formed on HDG steel. The corrosion resistance of the composite coatings, related to the pH value, contents of hydrated gel of silica and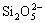 and post-sealing time, is also increased markedly.
and post-sealing time, is also increased markedly.
4) The corrosion resistance of the silicate improved phosphate coatings is increased in the following order: post-sealing>pretreatment>additives. The improved coatings with optimal corrosion resistance are obtained for phosphating 5 min and post-sealing with 5 g/L silicate solution for 10 min.
References
[1] Weng D, Jokiel P, Uebleis A, Boehni H. Corrosion and protection characteristics of zinc and manganese phosphate coatings [J]. Surf Coat Technol, 1997, 88(1/3): 147-156.
[2] Akhtar A S, Susac D, Glaze P, Wong K C, Wong P C, Mitchell K A R. The effect of Ni2+ on zinc phosphating of 2024-T3 Al alloy [J]. Surf Coat Technol, 2004, 187(2/3): 208-215.
[3] Wolpers M, Angeli J. Activation of galvanized steel surfaces before zinc phosphating—XPS and GDOES investigation [J]. Appl Surf Sci, 2001, 179(1/4): 281-291.
[4] NIU Li-yuan, LI Guang-yu, JIANG Zhong-hao, SUN Li-ping, HAN Dong, LIAN Jian-she. Influence of sodium metanitrobenzene sulphonate on structures and surface morphologies of phosphate coating on AZ91D [J]. Trans Nonferrous Met Soc China, 2006, 16(3): 567-571.
[5] Freeman D B. Phosphating and metal pre-treatment [M]. New York: Industrial Press, 1986: 134-139.
[6] Susac D, Leung C W, Sun X, Wong K C, Mitchell K A R. Comparison of a chromic acid and a BTSE final rinse applied to phosphated 2024-T3 aluminum alloy [J]. Surf Coat Technol, 2004, 187(2/3): 216-224.
[7] Shigeyoshi M, Masahiro Y. The role of chromate treatment after phosphating in paint adhesion [J]. Prog Org Coat, 1998, 33(1): 83-89.
[8] Aramaki K. The inhibition effects of chromate-free, anion inhibitors on corrosion of zinc in aerated 0.5 M NaCl [J]. Corros Sci, 2001, 43(3): 591-604.
[9] Hamdy A S. Corrosion protection of aluminum composites by silicate/cerate conversion coating [J]. Surf Coat Technol, 2006, 200(12/13): 3786-3792.
[10] Shi Xi-chang, Jarjoura G, Kipouros G J. Conversion coating treatment for AZ31 alloy in a permanganate-phosphate solution [J]. Magnesium Technol, 2006(2006): 273-280.
[11] Hamdy A S, Butt D P. Environmentally compliant silica conversion coatings prepared by sol-gel method for aluminum alloys [J]. Surf Coat Technol, 2006, 201(1/2): 401-407.
[12] Hamdy A S, Beccaria A M. Chrome-free pretreatment for aluminum composites [J]. Surf Interface Anal, 2002, 34(1): 160-163.
[13] LIN Bi-lan, LU Jin-tang, KONG Gang. Electrochemical behaviors of the phosphated/molybdate post-sealed hot-dip galvanized steel [J]. Journal of South China University of Technology (Natural Science Edition), 2007, 35(9): 113-118. (in Chinese)
[14] LIN Bi-lan, KONG Gang, LU Jin-tang, LIU Jun. Study of growth and corrosion resistance of zinc phosphate conversion coatings on hot dip galvanized steel [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(5): 800-806. (in Chinese)
[15] Sastri V S. Corrosion inhibitors [M]. Wiley: Chichester, 1998: 715-719.
[16] Parashar G, Bajpayee M, Kamani P K. Water-borne non-toxic high-performance inorganic silicate coatings [J]. Surface Coatings International Part B: Coatings Transactions, 2003, 86(3): 209-216.
[17] YANG Xi-zhen, YANG Wu. Electrochemical thermodynamics of metallic corrosion: Potential-pH diagrams and their applications [M]. Beijing: Chemical Industry Press, 1991: 210-212. (in Chinese)
(Edited by LI Xiang-qun)
Foundation item: Project(07BS405) supported by the Excellent Talents Foundation in Huaqiao Univeristy, China
Corresponding author: XU Yu-ye; Tel: +86-13665961191; E-mail: yuyexu@hqu.edu.cn