


Trans. Nonferrous Met. Soc. China 22(2012) 621-626
Modified carbothermal reduction method for synthesis of LiFePO4/C composite
YIN Yan-hong1, 2, LI Shao-yu3, YAN Lin-lin1, 2, ZHANG Hui-shuang1, 2, YANG Shu-ting1, 2
1. College of Chemistry and Environmental Science, Henan Normal University, Xinxiang 453007, China
2. Henan Engineering Research Center of Motive Power and Key Materials, Xinxiang 453007, China;
3. College of Physics and Information Engineering, Henan Normal University, Xinxiang 453007, China
Received 10 January 2011; accepted 15 January 2012
Abstract: With LiAc·2H2O as Li precursor, pure olivine phase LiFePO4/C was synthesized at a relatively low temperature (650 °C) and short sintering period (4 h) by molten salt carbothermal reduction method. Scanning electron micrograph shows that particle size of the product is about 1μm, smaller than that of the sample synthesized with Li2CO3 as Li precursor. Electrochemical measurements prove that LiFePO4/C obtained from LiAc·2H2O shows high capacity. The initial discharge capacities are 148 mA·h/g at 0.5C rate and 115 mA·h/g at 5C rate, respectively. After 50 cycles, the capacity retention ratios are 93% and 89% at 0.5C rate and 5C rate, respectively.
Key words: LiFePO4/C composite; molten salt; carbothermal reduction; β-cyclodextrin
1 Introduction
Recently, olivine-structured LiFePO4 proposed by PADHI et al [1] has received particular interest as a candidate cathode material for rechargeable lithium ion batteries. This kind of material has merits, such as high energy density, low cost, environmental friendliness and safety. However, the very poor electronic conductivity and Li-ion diffusion coefficient of LiFePO4 lead to its poor rate capability, which limits its industrial application to great extend. Therefore, research on LiFePO4 is mostly devoted to enhance its conductivity by metal doping [2,3], coating with the electronically conductive materials like carbon, metal and conductive polymer [4-6] or minimizing the particle size by modifying synthesis conditions [7,8].
As is known to all, synthesis methods would significantly affect the electrochemical performance of material. Up to now, several processing methods have been developed, such as high-temperature solid-state reaction, sol–gel reaction, hydrothermal method, co-precipitation and microwave heating [9-11]. Solid-state reaction is considered the suitable method for mass production of LiFePO4 because of the relatively simple processing procedure. However, the solid-state reaction has shortcomings, such as high energy consumption and poor uniformity of the product. Other soft-chemistry methods require complicated synthetic techniques, hard-controlled synthesis condition and relatively high cost of Fe(II) precursor. In recent years, the solid-state carbothermal reduction (CTR) method has been widely explored in the preparation of LiFePO4 [12,13]. This method has a lot of advantages, such as low cost raw materials, simple synthesis process and easy mass production application. However, as the traditional solid-state reaction, the uniformity of the product is hard to be controlled and the energy consumption is relatively high.
Recently, the molten salt method has been widely employed to manufacture cathode materials for lithium-based secondary batteries [14]. Small particles can be obtained at relatively low temperatures due to the high ion diffusion rates between reaction components in the molten media. But the traditional molten salt method must introduce NaCl [14], which acts as molten salt and must be washed off after the reaction. This will increase the complexity of the producing process. It is urgent to develop a simple, cheap and efficient method for the mass production of LiFePO4 cathode material.
In this work, with LiAc·2H2O as lithium source and molten media, β-cyclodextrin as carbon sources and reducing agent, a modified carbothermal reduction method (which is called as molten salt carbothermal reduction method) is used to produce LiFePO4 at a lower sintering temperature in a shorter reaction period. It is well known that the melting point of LiAc·2H2O is as low as 53-56 °C. During the reaction process, the molten LiAc·2H2O can offer liquid environment with high ion diffusion rate and strong dissolving capability, which is benefit for the ionic diffusion and can accelerate the reaction. Another merit is that the product does not need washing because LiAc·2H2O also acts as reactant. As comparison, the synthesis and characteristic of LiFePO4/C composite materials by ordinary carbothermal reduction route using Li2CO3 as lithium source are also presented.
2 Experimental
LiFePO4/C composites were prepared by the modified carbothermal reduction method using LiAc·2H2O (AR), NH4H2PO4 (AR) and Fe2O3 (AR) as starting materials. Stoichiometric LiAc·2H2O, NH4H2PO4, Fe2O3 and a certain amount β-cyclodextrin (9%, in mole fraction) were dissolved in de-ionized water and thoroughly mixed. After that, the mixture was dried in an oven at 80 °C for 8 h in air to form solid paste. Then the precursors were pressed into pellets and heated to 350 °C for 4 h and then to 550 °C, 600 °C and 650 °C for 4 h in nitrogen atmosphere, respectively. Samples with different temperatures of 550, 600 and 650 °C were named as a, b and c respectively. As comparison, LiFePO4/C composite materials were synthesized by ordinary carbothermal reduction method using the same pretreatment process. Among raw materials, only lithium source was changed to Li2CO3. The resulting precursors were sintered at 650 °C for 4 h and 700 °C for 10 h, which were nominated as samples d and e.
Thermal studies were carried out by means of thermogravimetric-differential analysis (TG-DTA) using a thermal analyzer system (Shimadzu DT-40) at a heating rate of 5 °C /min up to 700 °C in a N2 flow. XRD patterns were obtained from a Bruker AXS D8 X-ray diffractometer with Cu Kα radiation at 40 kV and 40 mA. SEM (AMARY-1000B) was used to observe the particle morphology.
Electrochemical performance of LiFePO4/C composites was characterized using CR2016 coin-type cell. The as-prepared powder was blended with acetylene and polyvinylidene fluoride (PVDF) with a mass ratio of 80:10:10, following the mixing with N- methylpyrolidinone (NMP) to form slurry. The slurry was then pasted onto an Al foil and the solvent was evaporated at 110 °C for 10 h under vacuum and finally pressed under 0.3 MPa. A disk cut from Al foil pasted with LiFePO4/C (about 0.02 mm thick) was used as the test electrode. Lithium metal was used as counter and reference electrodes. Ethylene carbonate (EC), dimethyl carbonate (DMC) and 1 mol/L LiPF6 were used as electrolyte. The coin cells were assembled in the glove box (ZKX3, Wuhan). Charge-discharge and cycling were carried out on a battery test system (CT2001A LAND, Wuhan). Normally the cells were cycled galvanostatically and the cut-off voltage was controlled between 2.5 and 4.2 V. The electrochemical impedance spectroscopy (EIS) and the cyclic voltammetry (CV) analyses were both carried out using a CHI660B electrochemical work station (Chenhua, Shanghai). The amplitude of AC signal was 5 mV over the frequency range of 0.1 Hz-105 Hz. The scan rate of CV was 0.01 mV/s in the voltage range of 2.5-4.3 V. All tests were performed at room temperature.
3 Results and discussion
Figure 1 shows the TG-DTA curves of the precursor containing LiAc·2H2O from room temperature to 700 °C with a heating rate of 5 °C/min in a N2 flow. It shows that there are various stages of mass losses. In the range of 50–160 °C, an initial mass loss and two weak endothermic peaks are observed. This continuous mass loss may correspond to the removal of physi-sorbed water, crystal water and melting of LiAc·2H2O, respectively. From 150 to 275 °C, there appear two mass loss steps (6.578% and 11.84%) and a strong endothermic peak in the DTA-TG curves, which can be ascribed to the thermal decomposition of LiAc and NH4H2PO4. Between 275 and 650 °C, the DTA plot shows two wide exothermic peaks with two mass losses of 9.539% and 8.223%, which can be attributed to the decomposition, carbonization, oxidation of β- cyclodextrin and crystallization of LiFePO4 [15-17].
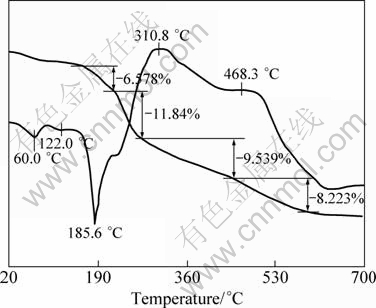
Fig. 1 TG-DTA profiles for precursor of LiFePO4/C using LiAc·2H2O as lithium source
There is no mass loss for further increase of temperature. Therefore, we choose three different synthesis temperatures, 550 °C, 600 °C and 650 °C to obtain LiFePO4/C composites.
The XRD patterns of LiFePO4/C composites sintered at different temperatures using the modified (a-c) and ordinary (d-e) carbothermal reduction methods are shown in Fig. 2. With the increase of temperature, the intensity of the diffraction peaks of LiFePO4 phase becomes stronger and stronger, indicating the increase of phase purity and crystallinity of LiFePO4. When the temperature is higher than 650 °C, all the diffraction patterns show a single and well-crystallized LiFePO4 phase with an olivine structure and a space group of Pnma. The diffraction peak intensity of Sample c is higher than that of Sample e (synthesized at 700 °C for 10 h [18, 19]) and is similar to that of Sample d. The results prove that the modified carbothermal reduction method can reduce the synthesis temperature and reaction time compared with the ordinary method. The reason is attributed to the high ion diffusion rate and strong dissolving capability of molten LiAc·2H2O.
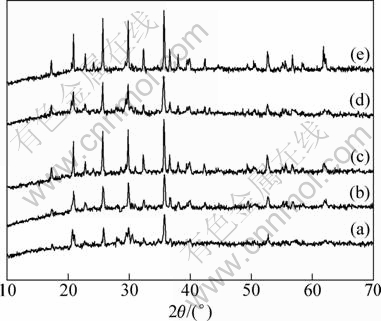
Fig. 2 XRD patterns of LiFePO4/C samples sintered at different temperatures using modified and ordinary solid state carbothermal reduction: (a) 550 °C, 4 h, LiAc as Li source; (b) 600 °C, 4 h, LiAc as Li source; (c) 650 °C, 4 h, LiAc as Li source; (d) 650 °C, 4 h, Li2CO3 as Li source; (e) 700 °C, 10 h, Li2CO3 as Li source
Scanning electron micrographs of LiFePO4/C composites synthesized by the modified carbothermal reduction method at 650 °C and ordinary one at 700 °C are presented in Fig. 3. It can be seen that particle size of LiFePO4/C composites synthesized by the modified method is much smaller than that of the ordinary one. The former with quasi-spheric shape shows a uniform and narrow size distribution of around 1 μm. However, the latter with irregular appearance shows a wide particle size distribution ranging from 1 to 5 μm. It is well known that in addition to crystallinity, the particle morphology, particle size and particle size distribution of cathode materials are of great importance to the performance of batteries. Smaller particle size and narrower particle size distribution are benefit for Li ion diffusion and improvement of its electrochemical performance.
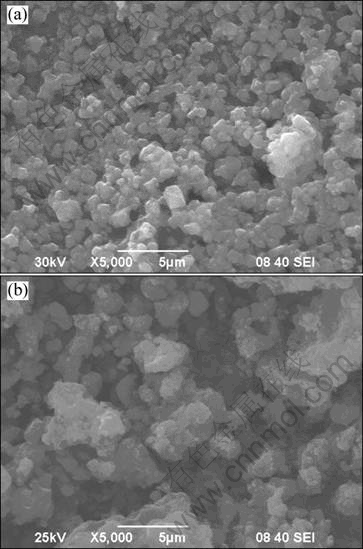
Fig. 3 SEM images of LiFePO4/C samples sintered at different temperatures using modified and ordinary solid state carbothermal reduction: (a) Sample c; (b) Sample e
Figures 4 and 5 show the charge and discharge curves of the LiFePO4/C electrodes at various current rates. For the LiFePO4/C composites synthesized by the modified method, the reversible capacity was 148 mA·h/g at 0.5C and 115 mA·h/g at 5C rate. While for the ordinary one, the reversible capacity was 122 mA·h/g at 0.5C and 61 mA·h/g at 5C rate. It should also be noticed that polarization of the LiFePO4/C composites synthesized by the modified method is slighter than that synthesized by the ordinary method at 5C rate. This can be attributed to the relatively good particle morphology, small particle size and narrow particle size distribution of the former. During the charge-discharge processes, the diffusion of Li ion is more harder inside the particle than outside. Therefore the utilization ratio of Li ion in large particles is lower than that in small particles, especially when the current density is not too low. This can explain why the discharge capacity of LiFePO4/C synthesized by the modified method is higher than that of samples synthesized by the ordinary method.
Figure 6 shows the CV profiles of LiFePO4/C electrode synthesized by modified and ordinary carbothermal reduction methods sintered at 650 °C and 700 °C, respectively. It can be seen that the anodic and cathodic peaks on CV curve of Sample c are more symmetrical and sharper than those of Sample e, and the amplitude of the peak current of the former is almost twice that of the latter. For Sample c the peak potential difference between cathodic and anodic peaks is 0.27 V, whereas for Sample e, it is 0.34 V. The well-defined peaks and the smaller peak potential difference on CV curve of Sample c suggest the higher reversibility of the electrode reaction.
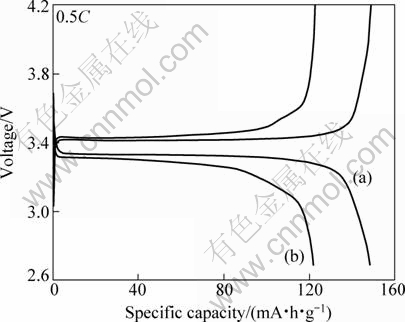
Fig. 4 Charge-discharge profiles of LiFePO4/C samples prepared by modified and ordinary solid state carbothermal reduction at 0.5C rate: (a) Sample c; (b) Sample e
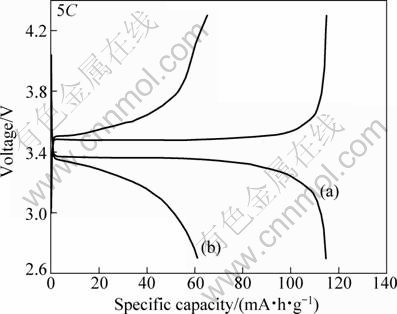
Fig. 5 Charge-discharge profiles of LiFePO4/C samples prepared by modified and ordinary solid state carbothermal reduction at 5C rate: (a) Sample c; (b) Sample e
AC impedance of the electrodes with different sintering time using modified and ordinary carbothermal reduction methods is shown in Fig. 7. All profiles exhibit a semicircle in the high frequency and a straight line in the low-frequency region. The values of the cross point of the semicircle on the horizontal axis represent the charge transfer resistance (Rct). The straight line represents the Warburg impedance, which is associated with the lithium-ion diffusion in the inner of LiFePO4 particles. The smaller numerical value of the diameter of the semicircle along the Z′ axis means smaller charge-transfer resistance and thus better reaction kinetics of lithium ion. From Fig. 7 it is obvious that Rct of Sample c is remarkably smaller than that of Sample e, indicating the increase of conductivity of Sample c, which explains its excellent electrochemical performance.
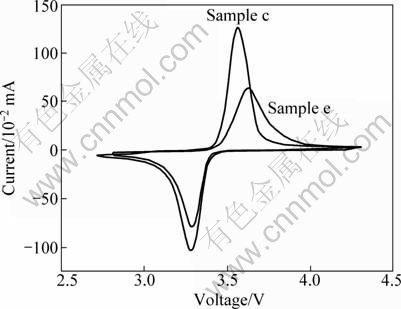
Fig. 6 Cyclic voltammograms of the LiFePO4/C samples prepared by the modified and ordinary solid state carbothermal reduction methods
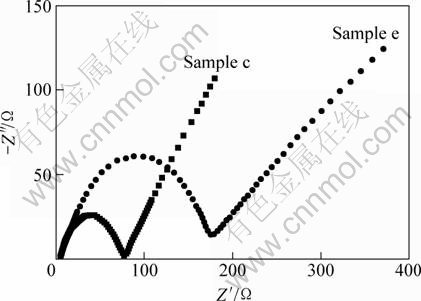
Fig. 7 AC impedance plots of LiFePO4/C samples prepared by modified and ordinary solid state carbothermal reduction methods
Figure 8 shows the cycling performance of LiFePO4/C samples at different current rates. It can be clearly seen that sample c shows much better cycling ability. At 0.5C rate, the initial discharge capacity of Sample c is 148 mA·h/g, and after 50 cycles it is 137 mA·h/g with a capacity retention up to 93%. For Sample e, the initial discharge capacity is 122 mA·h/g and the capacity retention is only 86%. At 5C rate, the capacity retentions of Samples c and e are 89% and 81%, respectively. According to the results of WANG et al [20], the formation of cracks in LiFePO4 particles after cycling would lead to poor electric contact and capacity fading. From literature, large particles with higher internal strain during Li-extraction/insertion process, crack more easily than smaller ones. So, the relatively small particle size and high electric conductivity explain the high capacity and excellent cycling performance of Sample c.
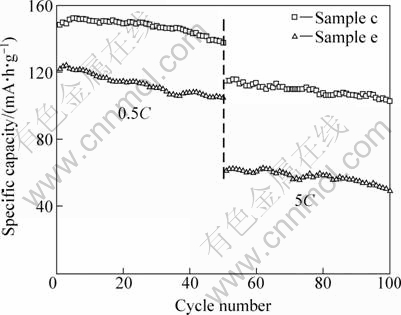
Fig. 8 Cycling performance of LiFePO4/C samples at different current rates
4 Conclusions
1) LiFePO4/C composites with pure olivine phase were synthesized using the modified carbothermal reduction method with LiAc·2H2O as Li-precursor. The sintering temperature and time (650 °C, 4 h) are reduced compared with the ordinary method using Li2CO3 as Li source (usually higher than 700 °C for at least 10 h).
2) As cathode material for Li-ion batteries, it can deliver a high capacity of 148 mA·h/g at 0.5C rate and 115 mA·h/g at 5C rate. After 50 cycles, the capacity retention ratios are 93% and 89% at 0.5C rate and 5C rate, respectively.
References
[1] PADHI A K, NANJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. J Electrochem Soc, 1997, 144: 1188-1194.
[2] SUN C S, ZhOU Z, XU Z G, WANG D G, WEI J P, BIAN X K, YAN J. Improved high-rate charge/discharge performances of LiFePO4/C via V-doping [J]. Journal of Power Sources, 2009, 193: 841-845.
[3] CHUNG S Y, BLOKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J]. Nat Mater, 2002, 1: 123-128.
[4] CHEN Zhao-yong, ZHU Hua-li, ZHU Wei, ZHANG Jian-li, LI Qi-feng, Electrochemical performance of carbon nanotube-modified LiFePO4 cathodes for Li-ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(4): 614-618.
[5] WU Ling, WANG Zhi-xing, LI Xin-hai, LI Ling-jun, GUO Hua-jun, ZHENG Jun-chao, WANG Xiao-juan. Electrochemical performance of Ti4+-doped LiFePO4 synthesized by co-precipitation and post-sintering method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 814-818.
[6] BAI Yong-mei, QIU Peng, WEN Zhong-liu, HAN Shao-chang. Synthesis and electrochemical properties of LiFePO4/PANI composites [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(8): 1572-1577. (in Chinese)
[7] PROSINI P P, CAREWSKA M, SCACCIA S, WISNIEWSKI P, PASQUALI M. Long-term cyclability of nanostructured LiFePO4 [J]. Electrochim Acta, 2003, 48: 4205-4211.
[8] KIM H S, CHO B W, CHO W. Cycling performance of LiFePO4 cathode material for lithium secondary batteries [J]. J Power Sources, 2004, 132: 235-239.
[9] MI C H, CAO Y X, ZHANG X G, ZHAO X B, LI H L. Synthesis and characterization of LiFePO4/(Ag+C) composite cathodes with nano-carbon webs [J]. Powder Technology, 2008, 181: 301-306.
[10] PARK K S, SON J T, CHUNG H T, KIM S J, LEE C H, KIM H G. Synthesis of LiFePO4 by co-precipitation and microwave heating [J]. Electrochem Commun, 2003, 5: 839-842.
[11] YANG S F, ZAVALIJ P Y, WHITTINGHAM M S. Hydrothermal synthesis of lithium iron phosphate cathodes [J]. Electrochem Commun, 2001, 3: 505-508.
[12] WANG L, LIANG G C, OU X Q, ZHI X K, ZHANG J P, CUI J Y. Effect of synthesis temperature on the properties of LiFePO4/C composites prepared by carbothermal reduction [J]. Journal of Power Sources, 2009, 189: 423-428.
[13] ZHONG Mei-e, ZHOU Zhi-hui, ZHOU Zhen-tao. Effects of Fe3+ sources on structure and properties of LiFePO4/C prepared by carbothermal reduction method [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(8): 1462-1467. (in Chinese)
[14] CHEN Zhao-yong, ZHU Wei, ZHU Hua-li, ZHANG Jian-li, LI Qi-feng. Electrochemical performances of LiFePO4/C composites prepared by molten salt method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(5): 809-813.
[15] LIU X H, ZHAO Z W. Synthesis of LiFePO4/C by solid–liquid reaction milling method [J]. Powder Technology, 2010, 197: 309-313.
[16] LEE S B, JANG I C, LIM H H, ARAVINDAN V, KIM H S, LEE Y S. Preparation and electrochemical characterization of LiFePO4 nanoparticles with high rate capability by a sol–gel method [J]. J Alloy Compd, 2010, 491: 668-672.
[17] ZHI X K, LIANG G C, WANG L, OU X Q, GAO L M, JIE X F. Optimization of carbon coatings on LiFePO4: Carbonization temperature and carbon content [J]. J Alloys Compd, 2010, 503: 370-374.
[18] LIU H, XIE J Y, WANG K. Synthesis and characterization of LiFePO4/(C+Fe2P) composite cathodes [J]. Solid State Ionics, 2008, 179: 1768-1771.
[19] ZHAO B, JIANG Y, ZHANG H J, TAO H H, ZHANG M Y, JIAO Z. Morphology and electrical properties of carbon coated LiFePO4 cathode materials [J]. Journal of Power Sources, 2009, 189: 462-466.
[20] WANG D Y, WU X D, WANG Z X, CHEN L Q. Cracking causing cyclic instability of LiFePO4 cathode material [J]. Journal of Power Sources, 2005, 140: 125-128.
改进的碳热还原法合成LiFePO4/C材料
尹艳红1, 2,李少玉3,闫琳琳1, 2,张会双1, 2,杨书廷1, 2
1. 河南师范大学 化学与环境科学学院,新乡 453007;
2. 河南省动力电源及关键材料工程技术研究中心,新乡 453007;
3. 河南师范大学 物理与信息工程学院,新乡 453007
摘 要:采用LiAc·2H2O作为锂源,利用熔盐碳热还原方法在较低的烧结温度和较短的烧结时间内(650 °C,4 h)合成纯相LiFePO4/C材料。扫描电镜照片显示这种方法合成的材料粒径大约为1 μm,小于用Li2CO3作为锂源合成的材料。电化学测试表明,采用LiAc·2H2O作为锂源合成的材料表现出了高的放电容量和良好的倍率循环性能:在0.5C和5C倍率下,其首次放电容量分别为148 mA·h/g 和115 mA·h/g;50次循环后,容量保持率分别为93% 和89%。
关键词:LiFePO4/C;熔盐;碳热还原;β-环糊精
(Edited by LI Xiang-qun)
Foundation item: Project (21001041) supported by the National Natural Science Foundation of China; Project (102300410256) supported by Henan Province Foundation and Advanced Technology Research Program, China; Project (102102210183) supported by the Key Scientific and Technological Research Project of Henan Province, China; Project (2011B480005) supported by the Natural Science Research Project of Henan Province, China
Corresponding author: YIN Yan-hong; Tel/Fax: +86-373-3326439; E-mail: yinyh@163.com
DOI: 10.1016/S1003-6326(11)61223-5