Cu和Mg对2014铝合金触变成形性和力学性能的影响
来源期刊:中国有色金属学报(英文版)2020年第2期
论文作者:M. A. M. ARIF M. Z. OMAR Z. SAJURI M. S. SALLEH
文章页码:275 - 287
关键词:JMatPro;锻造2014铝合金; 触变成形;半固态加工;力学性能
Key words:JMatPro; wrought 2014 aluminium alloy; thixoformability; semisolid processing; mechanical properties
摘 要:触变成形是使金属在半固态时发生变形的一种加工方法。该工艺的优点包括具有良好的表面光洁度,细小的显微组织和优越的力学性能,然而,该工艺主要是用铝铸件生产零件,没有充分利用其真正潜力。因此,可用热力学模拟进行合金成分设计。研究减少铜含量和增加硅、镁含量对2014铝合金触变成形的影响,包括模拟和实验验证两部分。结果表明,增加合金中硅含量和降低铜含量,可以降低合金的凝固区间温度,扩大特定液相分数之间的工作温度窗口,这对该工艺有利。高的固溶温度导致Mg2Si化合物的分解。Mg含量的增加导致致密π-Al8FeMg3Si6相的形成和尖片状结构β-Al5FeSi相的减少,提高合金的强度。后续T6热处理进一步提高改性合金的强度。
Abstract: Thixoforming is a processing method that deforms metal in a semisolid state. The advantages of this process include the production of parts with good surface finish, fine microstructures and superior mechanical properties. However, the process mostly produces parts from aluminium cast grades, thereby not fully utilising the true potential of this method. Hence, thermodynamic modelling can be used to formulate alloy compositions that favour this processing method. Here, the effects of reducing copper content and increasing silicon and magnesium contents on the thixoformability of aluminium alloy 2014 were presented. The work consists of both the modelling and experimental validation. Results showed that by increasing Si and decreasing Cu content in the alloy, the solidification interval temperature was decreased and the temperature working window between the stipulated liquid fractions was widened, two of the characteristics favouring the process. A high solid-solution temperature employed resulted in the dissolution of unfavourable Mg2Si compound. An increase in Mg content used also resulted in the formation of the compact π-Al8FeMg3Si6 phase and the decrease in the amount of the sharp and plate-like structure of the β-Al5FeSi phase, improving the strength of the modified alloy. Subsequent T6 heat treatment successfully further increased the strength of the modified alloy.
Trans. Nonferrous Met. Soc. China 30(2020) 275-287
M. A. M. ARIF1, M. Z. OMAR1, Z. SAJURI1, M. S. SALLEH2
1. Centre for Materials Engineering and Smart Manufacturing (MERCU), Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600, Selangor, Malaysia;
2. Department of Manufacturing Process, Faculty of Manufacturing Engineering, Universiti Teknikal Melaka, Hang Tuah Jaya, 76100 Durian Tunggal, Melaka, Malaysia
Received 9 April 2019; accepted 12 November 2019
Abstract: Thixoforming is a processing method that deforms metal in a semisolid state. The advantages of this process include the production of parts with good surface finish, fine microstructures and superior mechanical properties. However, the process mostly produces parts from aluminium cast grades, thereby not fully utilising the true potential of this method. Hence, thermodynamic modelling can be used to formulate alloy compositions that favour this processing method. Here, the effects of reducing copper content and increasing silicon and magnesium contents on the thixoformability of aluminium alloy 2014 were presented. The work consists of both the modelling and experimental validation. Results showed that by increasing Si and decreasing Cu content in the alloy, the solidification interval temperature was decreased and the temperature working window between the stipulated liquid fractions was widened, two of the characteristics favouring the process. A high solid-solution temperature employed resulted in the dissolution of unfavourable Mg2Si compound. An increase in Mg content used also resulted in the formation of the compact π-Al8FeMg3Si6 phase and the decrease in the amount of the sharp and plate-like structure of the β-Al5FeSi phase, improving the strength of the modified alloy. Subsequent T6 heat treatment successfully further increased the strength of the modified alloy.
Key words: JMatPro; wrought 2014 aluminium alloy; thixoformability; semisolid processing; mechanical properties
1 Introduction
Thixoforming, also known as semisolid metal (SSM) processing that combines casting and forging, produces near-net-shape metal components [1]. Thixoforming has the advantage of producing metals with good surface finish, fine and uniform microstructure with reduced porosity, and superior mechanical properties [2-5]. The main requirement of thixoforming is the feedstock material with thixotropic properties. These alloys can stand stiff like solid if undisturbed and flow like liquid when they are sheared [6]. These properties can be achieved, if the microstructure of the alloys consists of a solid spheroidal structure in a liquid matrix [7-9]. Various methods can be used to generate such structures, including strain-induced melt activation (SIMA), magnetohydrodynamic stirring (MHD), recrystallization and partial melting (RAP), and cooling slope (CS) [10-15].
Several studies on thixoforming were conducted on a considerable quantity of alloys of aluminium (Al) with various compositions and semisolid ranges. However, only several commercial alloys were employed for thixoforming. It is known that Al-Si-Cu is from the cast Al alloys that are applicable to SSM processing which possesses acceptable mechanical strength and fluidity [16-18]. Therefore, the range of Al alloys utilized for thixoforming must be extended. The relationship between the liquid fraction and the temperature is one of the parameters which can be used as a reference for identification of alloy compositions that are suitable for thixoforming. The amount of liquid at the highest ‘knee’ on the liquid fraction versus temperature curve represents the amount of eutectic phases in the alloy structure. The highest ‘knee’ is defined as the temperature at which the eutectic phases completely melt and α(Al) phase starts to melt [19]. High performance wrought 2014 Al alloy is one of the strongest available Al alloys, which exhibits a high degree of hardness. However, practical experiences in thixoforming have shown that wrought 2014 Al alloy is susceptible to hot tearing due to the wide solidification interval temperature (131 °C) of this material. Thixoforming succeeds when the solidification interval temperature of the alloy is less than 130 °C. In addition, the amount of eutectic phases for wrought 2014 Al alloy is below the thixoforming range (the highest ‘knee’ occurs at a liquid fraction value less than 10 wt.%). However, this process is normally performed at a liquid fraction value between 30% and 50%. This shows that the alloy composition adjustment is needed to increase the amount of eutectic phases at the highest ‘knee’ point and decrease the solidification interval temperature. Based on the previous investigation, the amount of eutectic phases at the highest ‘knee’ point can be increased and the solidification interval can be decreased by adding silicon (Si) to wrought 2014 Al alloy. This is the reason why cast-grade Al alloys have high Si content and the casting process needs short solidification interval temperature. Simultaneously, the addition of Si to Al improves fluidity and provides good casting characteristics due to the formation of Al-Si eutectic [19].
Thermodynamic prediction software packages such as Thermo-Calc, ChemSage, MTDATA and Java-based Materials Properties (JMatPro) can be used to evaluate the thixoformability of existing alloys or to identify new alloy compositions suitable for thixoforming. Some researchers reported the potential of thermodynamic predictions for identifying thixoformable compositions in Al alloy. MACIEL CAMACHO et al [20] investigated the effect of the alloying elements on liquid fraction evolution during solidification and the phases in equilibrium for wrought Al 7xxx series Al-Zn- Mg-Cu system. In 2005, LIU et al [19] investigated the effects of adding copper (Cu) to cast alloy A356 and adding Si to wrought 2014 Al alloy on their liquid fraction versus temperature curve. Both of thermodynamic predictions were carried out using a commercial software package, MTDATA, developed by the National Physical Laboratory (NPL). ZOQUI et al [21] investigated the thixoformability of Al-Si alloys with various percentages of Si by using Thermo-Calc simulation software.
Although the effects of alloying elements on the thixoformability of Al alloys have been frequently reported in previous studies, the effects of decreased Cu amount and added Mg on the thixoformability criteria of wrought 2014 Al alloy still lack research. In the present study, the thixoformability of wrought 2014 Al alloy was investigated as the results of decreased Cu amount and added Mg. Si was also added to the alloy to increase the amount of eutectic phases at the highest ‘knee’ and decrease the solidification interval temperature. JMatPro, a thermodynamic prediction software package (Sente Software Ltd.) was used to predict the liquid fraction profiles and phases under Scheil condition. In addition, the effects of T6 heat treatment on the mechanical properties and phase transformation of the thixoformed alloy were examined.
2 Experimental
JMatPro, a thermodynamic prediction software package (by Sente Software Ltd.), was employed as a guide in order to thermodynamically model the development of the thixoforming alloy. The modified Al alloy utilized in this study with the calculated chemical composition was prepared by means of gravity casting method. Furthermore, the chemical composition of the alloy was inspected via the X-ray fluorescence (XRF) method and was compared to the standard wrought 2014 Al alloy composition in Table 1. The liquidus, solidus, and liquid fraction profiles were experimentally determined by means of differential scanning calorimetry (DSC) and were equated with the JMatPro calculated data. The DSC analysis was executed by the Netzsch-STA (TG-DSC) 449 F3 simultaneous thermogravimeter in a nitrogen (N2) atmosphere chamber, while the scanning rate was 10 °C/min.
Table 1 Chemical compositions of alloys studied (wt. %)

The alloy billets with non-dendritic structure were prepared by means of CS casting method. Primarily, the alloy ingots were melted in an argon (Ar) atmosphere through a resistance furnace before being poured (at a pouring temperature of 650 °C) onto the CS plate (cooling length of 400 mm and tilt angle of 60°). Furthermore, the alloy billets were then gathered in a pre-heated cylindrical stainless-steel mould (150 °C). The schematic of the CS casting apparatus equipped with a resistance furnace is illustrated in Fig. 1. The optimum CS factors such as tilt angle, pouring temperature, and cooling length were attained through the methods reported by SALLEH et al [23]. Subsequently, the alloy billets were thixoformed by a hydraulic cylinder press that delivered a load of 20 kN and possessed a maximum compression velocity of 85 mm/s, as displayed in Fig. 2. Next, the billets were promptly reheated (130 °C/min) into their semisolid state at 570 °C for 5 min. This was performed in order to inhibit the grain growth prior to thixoforming into a preheated stainless steel die (300 °C). The process of heating was conducted via a high-frequency induction coil (30-80 kHz, 35 kW) where it was stationed under the die. Moreover, the heating was checked by means of a calibrated K-type thermocouple. In order to avoid the oxidation during the thixoforming, Ar gas flowed at 2.5 L/min in the heating chamber.

Fig. 1 Schematic of CS casting apparatus

Fig. 2 Schematic of thixoforming machine
Furthermore, The T6 heat treatment was executed on few of the thixoformed samples with the aim of improving their mechanical properties, while the T6 heat treatment consisted of solid solution treatment at 525 °C for 8 h followed by warm water quenching at 60 °C. It must be mentioned that the quenched samples were aged at 155 °C for 4 h.
The observation on the microstructural characterisation was performed by means of an Olympus optical microscope once the surface of the samples was finished and was etched in Keller’s agent for 20 s. The elemental analyses of the microstructures were obtained via a Carl Zeiss (EvoMa10) scanning electron microscope (SEM) with attachments for energy dispersive X-ray (EDX) spectroscopy. Moreover, the X-ray diffraction (XRD) analysis was conducted in order to verify that the phases were present in the as-thixoformed and thixoformed-T6 alloy. The XRD analysis was performed via a Bruker D8-Advance X-ray diffractometer with nickel (Ni) filtered Cu Kα radiation at a scanning rate of 2 (°)/min. For the strength measurements, the tensile test specimens of the as-thixoformed and thixoformed-T6 alloys were obtained in accordance to the ASTM: E8M and were analysed at room temperature through a 100 kN Zwick Roell universal testing machine (UTM). Micro-hardness measurements were obtained from the as-thixoformed and thixoformed- T6 samples after grinding and polishing in order to attain a flat mirror-like surface. Ten micro-hardness readings were obtained from each sample by utilizing Shimadzu micro-hardness Vickers tester, HMV-2000 equipped with a square-based pyramid- shaped diamond indenter. It must be mentioned that a load of 1 N was applied for 10 s at room temperature.
3 Results and discussion
3.1 Thermodynamic prediction
The effects of compositional variations, particularly the effects of added Si and Mg and decreased Cu amount on the thixoformability of wrought 2014 Al alloy were examined using the JMatPro simulation software. Figure 3 shows the liquid fraction versus temperature as predicted by this software for wrought 2014 Al alloy and 2014 alloy modified with different amounts of Si. The prediction of the liquid fraction versus temperature relationship for wrought 2014 Al alloy (Al-0.8Si- 4.5Cu-0.5Mg-0.8Mn-0.7Fe-0.25Zn) is also present. The curve in Fig. 3 shows that wrought 2014 Al alloy has a wide solidification interval temperature which is estimated to be approximately 132 °C. Solidification interval refers to the temperature interval between the liquidus and solidus. However, the wide solidification range (more than 130 °C) can contribute to hot tearing and being susceptible to the formation of porosity of the material. Aside from providing good casting characteristics, the addition of Si in wrought 2014 Al alloy also decreased the solidification interval and was able to increase the amount of eutectic phases at the highest ‘knee’ point. Notably, the liquid formation of the materials tends to be controllable if the amount of eutectic phase in the structure is 0.30-0.50 of liquid fraction [19]. The prediction results showed that the solidification interval decreased and the amount of eutectic phases at the highest ‘knee’ point increased significantly by adding Si to wrought 2014 Al alloy. When Si content in the wrought 2014 Al alloy increased from 0.8 to 5 wt.%, the solidification interval decreased from 132 to 106 °C. Moreover, the amount of eutectic phases (at the highest ‘knee’ point) increased from 0.09 to 0.42. Therefore, the modified Al alloy (2014 Al alloy but with 5 wt.% Si) was likely to be more thixoformable than wrought 2014 Al alloy.
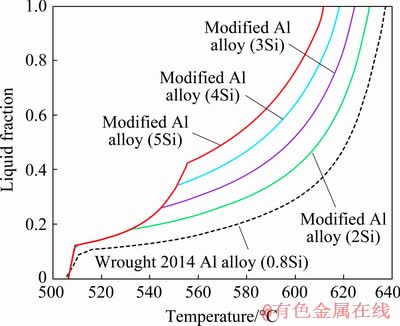
Fig. 3 Liquid fraction versus temperature for wrought 2014 Al alloy and 2014 alloy modified with different amounts of Si, calculated using JMatPro simulation software
The addition of Cu in Al-Si-Mg alloys provided a substantial increase in the strength of the material and facilitated the precipitation hardening due to the formation of CuAl2 phase and other intermetallic compounds [24]. However, the addition of Cu can reduce ductility and resistance to corrosion and hot tearing. It also sacrifices the castability of the alloy [25]. Therefore, further thermodynamic prediction was performed to investigate the effect of decreased Cu amount on the thixoformability of alloy 2014 modified with 5 wt.% Si. Figure 4 shows the changes of solidification interval temperature, amount of eutectic phases at the highest ‘knee’ point and processing window between 0.3 and 0.5 of liquid fraction with decreased Cu amount from Al-5Si- 4.5Cu-0.5Mg-0.8Mn-0.7Fe-0.25Zn alloy. As a result, decreased Cu content from 4.5 to 0.25 wt.% led to decreased solidification interval from 106 to 91 °C and the amount of eutectic phases (at the highest ‘knee’ point) decreased from 0.42 to 0.33 of liquid fraction. Meanwhile, the solidus and liquidus temperatures increased from 506 to 534 °C and from 612 to 625 °C, respectively. Simultaneously, the working window between 0.3 and 0.5 of liquid fraction (ΔT0.3-0.5) was enlarged from 20 to 31 °C. In terms of the thixoformability specification, the modified alloy (with 5 wt.% Si and 0.25 wt.% Cu) was expected to be the most suitable material for thixoforming.
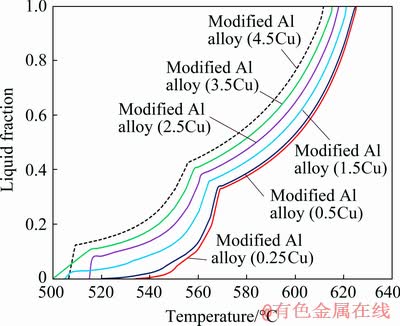
Fig. 4 Effect of decreased Cu amount on liquid fraction versus temperature for modified Al alloy (Al-5Si- 4.5Cu-0.5Mg-0.8Mn-0.7Fe-0.25Zn), calculated using JMatPro simulation software
Besides Cu, Mg is usually added to Al–Si–Cu casting alloys as an alloying element to enhance the mechanical properties of the alloys [26]. In the solution of Al-Si-Cu-Mg alloys, Mg and Si tend to combine together to form meta-stable compound of Mg2Si with a very high degree of hardness (HV 4500). This very high degree of hardness of Mg2Si particles improved the mechanical properties of the alloys due to the precipitation of very fine Mg2Si particles during age hardening of T6 heat treatment [16,27]. The effects of added Mg on the thixoformability of the modified alloy (with 5 wt.% Si and 0.25 wt.% Cu) were then thermodynamically calculated using the JMatPro software. Figure 5 shows the prediction of the liquid fraction for Al-5Si-0.25Cu-xMg-0.8Mn-0.7Fe-0.25Zn with different percentages of Mg (x=0.5, 0.8 and 1.2 wt.%). Adding Mg has no significant effects on the amount of eutectic phases at the highest ‘knee’ point and solidification interval. However, the highest ‘knee’ shifted to the left when the Mg content increased from 0.5 to 1.2 wt.%. Therefore, the temperature to melt completely the eutectic phases is slightly decreased from 570 to 562 °C. Table 2 lists the solidification parameters for wrought 2014 Al alloy and modified Al alloys given by JMatPro software corresponding to the liquid fraction versus temperature curves as shown in Figs. 3, 4 and 5, respectively.

Fig. 5 Effect of added Mg on liquid fraction versus temperature for modified Al alloy (Al-5Si-0.25Cu- 0.5Mg-0.8Mn-0.7Fe-0.25Zn), calculated using JMatPro simulation software
Table 2 Solidification parameters given by JMatPro for wrought 2014 Al alloy and modified Al alloys

Figure 6 shows the phase equilibrium diagram for Al-5Si-0.25Cu-xMg-0.8Mn-0.7Fe-0.25Zn alloy with different percentages of Mg (x=0.5, 0.8 and 1.2 wt.%) obtained using the JMatPro software. The diagram showed the presence of intermetallic phases (Si, Mg2Si, β-Al5FeSi and π-Al8FeMg3Si6) which were formed when alloying elements such as Mg and iron (Fe) were added into the Al-Si alloy. According to SAMUEL et al [28], the π-Al8Fe- Mg3Si6 phase transforms from β-Al5FeSi phase when the amount of Mg reaches 0.35 wt.%. The mass fraction and size of Mg2Si and π-Al8FeMg3Si6 phases increase with increased Mg content [28-30]. The π-Al8FeMg3Si6 phase has a Chinese-script-like or blocky morphology, whereas the β-Al5FeSi phase has a sharp and plate-like structure. The sharp and plate-like structure of the β-Al5FeSi phase deteriorated the mechanical properties of the Al alloys. However, the π-Al8FeMg3Si6 phase was less deteriorated than the β-Al5FeSi phase due to its compact morphology [31]. To achieve good mechanical properties, the β-Al5FeSi phase should be avoided or minimised. Based on the phase equilibrium diagram, as shown in Fig. 6, the π-Al8FeMg3Si6 phase is the dominant phase whose content increased with increased Mg content. The content of the π-Al8FeMg3Si6 phase significantly increased at about 5 wt.%, whereas that of the β-Al5FeSi phase decreased to 0.018 wt.% when the Mg content is 1.2 wt.%.

Fig. 6 Phase equilibrium diagrams for Al-5Si-0.25Cu- xMg-0.8Mn-0.7Fe-0.25Zn alloys with different percentages of Mg by JMatPro: (a) 0.5 wt.% Mg; (b) 0.8 wt.% Mg; (c) 1.2 wt.% Mg
The π-Al8FeMg3Si6 particles were hard to dissolve, whereas Mg2Si particles were relatively easy to dissolve [32]. To realise fully the ageing phase of alloys, the phases containing Mg and Cu elements should be given a complete dissolution during the solution treatment. The calculated data displayed in Fig. 4 indicate that higher Cu content provides lower solidus temperature. Consequently, the Al-Si-Cu-Mg alloys with a high Cu content were unable to be solution-treated at high temperature attributed to the possibility of melting the Cu-containing phases. Therefore, the phases containing Mg was not dissolved and the optimal solution treatment could not be obtained at a low solution treatment temperature [29]. In addition, SAMUEL et al [28] also stated that the strength value of an Al-Si-Cu alloy with the incorporation of Mg could not be enhanced via a solution treatment at 480 °C as a result of the lower concentration of alloying elements. However, at instances when the solution treatment temperature was raised to 500 °C, the strength of the alloy increased. In this study, it was observed that the Al- 5Si-0.25Cu-1.2Mg-0.8Mn-0.7Fe-0.25Zn alloy started to melt at 535 °C because of the non- formation of Cu-containing phases. Consequently, this modified Al alloy can be solution-treated at a high temperature in order to dissolve the Mg-containing phases.
3.2 Liquid fraction estimation
To evaluate the liquid fraction versus temperature curve calculated by JMatPro with experimental result, thermal analysis was conducted on the modified Al alloy (Al-5Si-0.25Cu-1.2Mg- 0.8Mn-0.7Fe-0.25Zn) by DSC experiment. Figure 7 illustrates the liquid fraction versus temperature curve by JMatPro and DSC heating curve, along with the corresponding liquid distribution. It can be observed that the highest ‘knee’ from DSC experiment occurred at a few degrees of temperature higher than the predictions. The liquid fraction at the highest ‘knee’ is estimated to contain 45% of liquid for DSC curve, whereas 34% of liquid for JMatPro. The discrepancy between the result from DSC and JMatPro prediction (Scheil calculation) probably was caused by a few factors. Scheil calculates a result for a bulk material but DSC result is obtained from a very small-scale sample. In addition, DSC results were obtained from uncertainty in the specification of the compositions and inhomogeneous distribution of the alloying elements in the alloy. However, the calculated result considers the ideal or perfect experiment. Kinetic factor also gives the highest ‘knee’ on the DSC curve occurred above the ‘knee’ in the predictions.
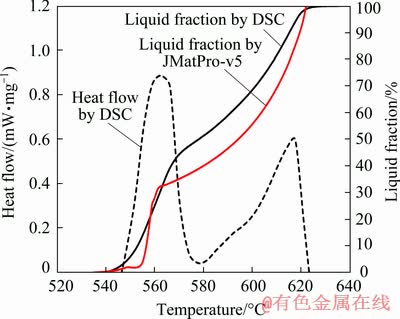
Fig. 7 DSC curve and liquid fraction profile for modified Al alloy
Notably, thixoforming is normally performed between 0.3 and 0.5 of the liquid fraction. In addition, the highest ‘knee’ on the curve of the liquid fraction versus temperature should occur in this liquid fraction range to achieve thixo- formability criteria [19]. In the present study, the DSC analysis indicated that the highest ‘knee’ of the modified Al alloy occurred in the range of SSM processing (liquid fraction of 0.45), i.e., within the targeted thixoformability criteria. Referring to the liquid fraction curve, the corresponding temperature for the liquid fraction of 0.45 is 570 °C.
3.3 Cooling slope casting and thixoforming
Figure 8(a) demonstrates the formation of dendritic microstructure in the as-cast sample of the modified Al alloy. The grey dendritic structure signifies the initial α(Al) phase, while the black inter-dendritic structure denotes the secondary eutectic phase. In addition, Fig. 8(b) displays the effects of the process of CS casting on the microstructure of the as-cast sample. The dendritic morphology changed to rosette as a result of the nucleation of primary particles. The crystal nuclei were generated in the melt while flowing along the cooling slope plate [14,22]. In addition, the effect of shear stress on the solid particles during the flow along the slope could also lead to the fragmentation of the dendrite arms, resulting in the non-dendritic structure [33].

Fig. 8 Microstructures of modified Al alloy: (a) As-cast; (b) After cooling slope casting process
Figure 9(a) shows the semisolid microstructure of the modified Al alloy that consists of solid spheroids in a liquid phase. The coarsening of the fine rosettes into spheroids and the melting of interdendritic structure occurred when the alloy produced by CS process was partially remelted at semisolid temperature (570 °C) for 5 min. Figure 9(b), on the other hand, shows the microstructure of the thixoformed alloy, exhibiting uniformly distributed near globular primary phase structure with hardly any micro pore observed.
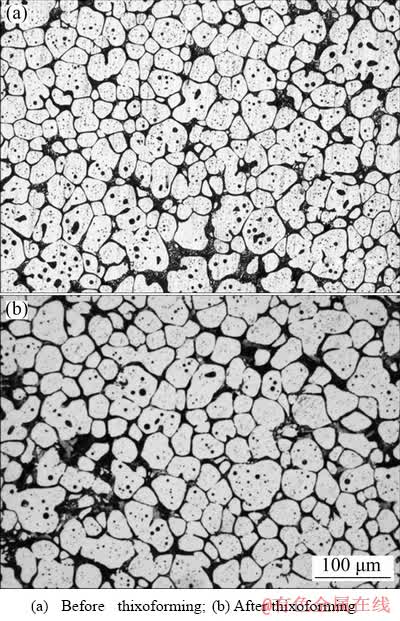
Fig. 9 Microstructures of modified Al alloy subjected to partial remelting process
3.4 Phase analysis
X-ray diffraction (XRD) analysis was performed to experimentally identify and validate the existing phase in the modified Al alloy. Figure 10 shows a portion of the experimental diffraction pattern for the thixoformed-modified Al alloys with different percentages of Mg content (x=0.5, 0.8 and 1.2 wt.%). The XRD patterns show the existence of the peaks of Al and Si phases as well as the small peaks for the intermetallic phases of Mg2Si and π-Al8FeMg3Si6 in the thixoformed alloy. The intensities of Si and Mg2Si phases for the thixoformed-modified Al alloy with 1.2 wt.% Mg content showed some increase after T6 heat treatment (solution-treated for 8 h at 525 °C and subsequently aged for 4 h at 155 °C).
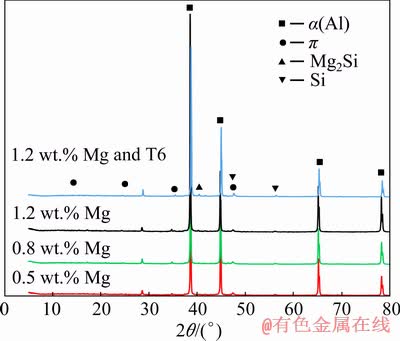
Fig. 10 XRD patterns for thixoformed Al alloys modified with different contents of Mg
Figure 11 shows SEM micrograph and EDX spectra of identified intermetallic phases for thixoformed sample of modified Al alloy. The microstructures consisted of the primary α(Al) phase surrounded by a very fine Al-Si eutectic phase. The intermetallics of Mg2Si and π-Al8FeMg3Si6 phases were also detected via EDX spot measurement and distinguished by different contrasts and morphologies. The very fine intermetallic phase of Mg2Si (black in colour) was concentrated and distributed around the primary α(Al) globules, whereas the intermetallic phase of π-Al8FeMg3Si6 exhibited large compact polyhedral morphology. Fe-rich phase plays an important role in improving the processing capabilities of the Al alloys which can avoid the material from sticking on the mould.

Fig. 11 SEM back scatter electron image and EDX spectra of modified Al alloy after thixoforming
The SEM micrograph and EDX spectra of the thixoformed sample of the modified Al alloy after the T6 heat treatment (solution-treated for 8 h at 525 °C and subsequently aged for 4 h at 155 °C) is shown in Fig. 12. The morphology of Si particles in the interdendritic regions changed from fibrous to spheroidal and increased in size after the T6 heat treatment. During solution treatment, the eutectic Si phase experienced necking and were broken down into fragments. The necking and breakdown phenomenon occurred due to the geometrical perturbations along the fibrous eutectic Si as heat was applied. The mechanism of this phenomenon is referred to as Rayleigh–Plateau instability theory. In this theory, the cylindrical body of fluid becomes unstable and breaks down to stable spherical droplets. As a result, the fragmented Si particles have completely transformed into the spheroidal form due to the spheroidisation and coarsening process. Prolonged solution treatment resulted in extensive coarsening of Si spheroids [29,34,35]. The effects of the T6 heat treatment on Mg-rich phase were also clearly visible in the micrograph. The Mg2Si particles appeared with black colour. The dissolution of Mg2Si compound in the matrix of α(Al) is the main reason for age hardening. During the solution treatment at high temperature (525 °C), Mg2Si phase formed during solidification starts to dissolve to achieve a high concentration and homogenous distribution in solid solution. Subsequently, the quenching process produced the supersaturated solid solution of Mg and Si in Al matrix. After the supersaturated solid solution was subjected to an artificial aging process, Mg2Si precipitated as finely dispersed particles. These particles were capable of increasing the hardness and strength of modified Al alloy. Precipitation sequence of Mg2Si is as follows [29,36]: Supersaturated solid solution → Guinier-Preston (GP) zones → β’(Mg2Si) → β(Mg2Si).
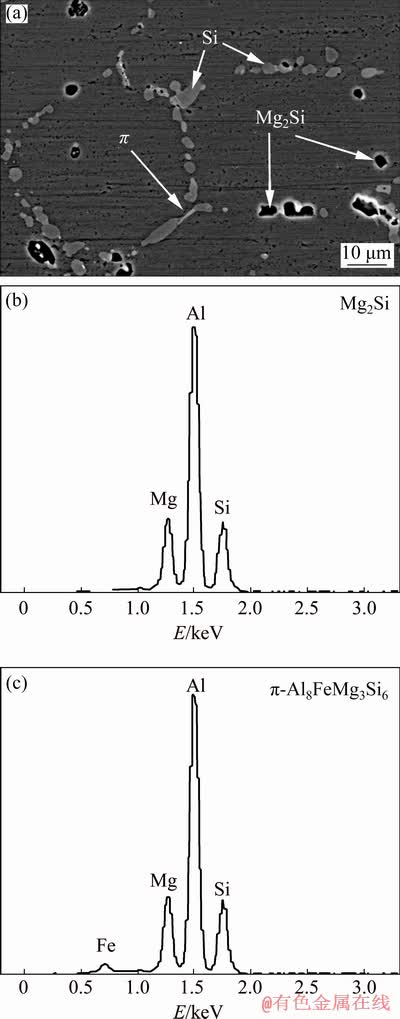
Fig. 12 SEM back scatter electron image and EDX spectra of modified Al alloy after thixoforming and T6 heat treatment
Furthermore, the addition up to 1.2 wt.% Mg into modified Al alloy also led to the replaced sharp edge of β-Al5FeSi phase to the compact π-Al8FeMg3Si6 phase (light grey colour) with less detrimental effects. However, π-Al8FeMg3Si6 phase was stable at elevated temperature as the Mg content in the modified Al alloy is increased. Hence, this phase is hard to dissolve and virtually unaffected by solution treatment [30,32].
3.5 Mechanical properties
Figures 13 and 14, respectively, show the tensile strength and the Vickers micro-hardness values of the modified Al alloy samples after thixoforming (before and after T6 heat treatment). The T6 heat treatment was conducted in order to examine the heat-treatability of the modified Al alloy after thixoforming. The heat treatment was proposed in order to enhance the mechanical properties of the alloy, specifically the hardness and tensile strength of the material. The obtained results after the T6 heat treatment indicated an increasing value of yield strength (YS) from (230±3.8) to (264±4) MPa and the ultimate tensile strength (UTS) from (263±4) to (306±3) MPa. In addition, the micro-hardness was increased from HV (107±6.8) to (126±5.5) subsequently after the T6 heat treatment. In addition, the enhancement in the mechanical properties after being subjected to the T6 heat treatment revealed the heat treatable behaviour of the modified Al alloy after thixoforming. This enhancement was assisted by the precipitation of Mg2Si phase which was formed as finely dispersed particles and the spheroidisation of the eutectic Si particles attributed to the T6 heat treatment, as discussed above. The precipitation of Mg2Si phase and spheroidisation of eutectic Si had a significant effect on the mechanical properties of the Al-Si-Cu-Mg alloys. The intermetallic phase of π-Al8FeMg3Si6 was hard to dissolve and was somewhat unaltered by the solution treatment. Nevertheless, this intermetallic phase with compact polyhedral morphology by means of the thixoforming process acts as a barrier to dislocation motion and exhibits a higher resistance to indentation [31]. As a comparison, Fig. 13 and Fig. 14 illustrate the typical tensile strength and the micro-hardness for automotive engine block alloys. In a study, TAVITAS-MEDRANO et al [37] reported that the alloy utilized in the preparation of the automotive engine blocks and cylinder heads must possess the typical YS of 250 MPa, UTS of 300 MPa, and micro-hardness of HV 100.

Fig. 13 Comparison of UTS and YS of engine block alloy and thixoformed-modified Al alloy before and after T6 heat treatment
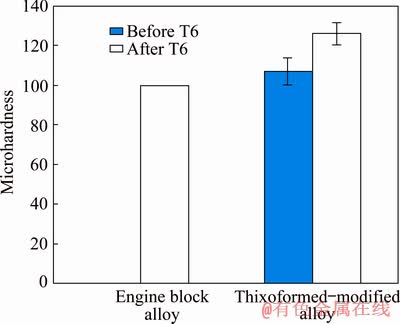
Fig. 14 Comparison of microhardness of engine block alloy and thixoformed-modified Al alloy before and after T6 heat treatment
3.6 Fracture behaviour
Figure 15 shows the SEM fractographs of the tensile fracture surface of the thixoformed- modified Al alloy samples before and after T6 heat treatment. The fractograph in Fig. 15(a) shows a brittle fracture with cleavage rupture in the thixoformed-modified Al alloy before T6 heat treatment. This fracture was transgranular where the cracks propagated through grains. After T6 heat treatment, the fractograph of the thixoformed- modified Al alloy in Fig. 15(b) showed a ductile fracture with a dimple structure. This dimpled fracture surface occurs as a result of microvoids coalescence. The Si particles and Mg2Si precipitates did not deform at the same rate as the matrix of α(Al) phase, resulting in the formation of the microvoids when stretched. Upon further plastic strain, the microvoids expanded and eventually coalesced before fracture [22]. The large number of microcracks in the micrographs proved that the occurrence of precipitation of the hard Mg2Si phase in the alloy after T6 heat treatment. As mentioned above, the precipitation of fine Mg2Si intermetallic phase contributed to the improvement in alloy strength.
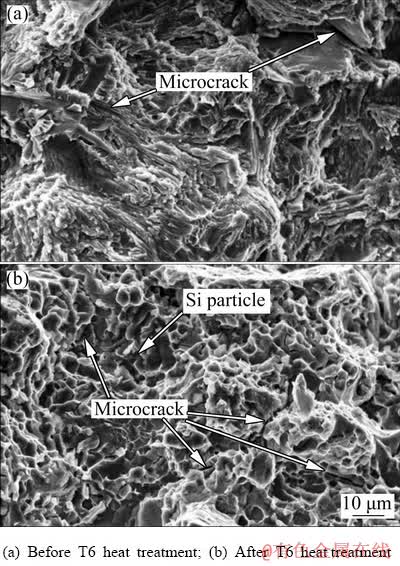
Fig. 15 SEM fractographs for thixoformed-modified Al alloy
4 Conclusions
(1) JMatPro was utilized in order to assess the effects of compositional deviations, predominantly the effects of decreased Cu amount and added Mg on the thixoformability of wrought 2014 Al alloy. The thermoformability criteria were focused on the solidification interval temperature and the amount of eutectic phases (the amount of liquid at the highest ‘knee’ on the liquid fraction versus temperature curve) of wrought 2014 and modified Al alloys. At instance, when the Si content in the wrought 2014 Al alloy was increased, the solidification interval temperature decreased and the amount of eutectic phases increased. However, the reduction in the Cu content led to the decrease in the value of the solidification interval temperature and the amount of eutectic phases. In addition, it was observed that the working window between 0.3 and 0.5 of liquid fraction was enlarged. Simultaneously, the solidus and liquidus increased due to the absence of Cu-containing phases. No significant effect was observed on the amount of eutectic phases and solidification interval temperature with the addition of Mg. However, the addition of Mg led to a decrease in the sharp and plate-like structure of the β-Al5FeSi phase that deteriorated the mechanical properties of the alloy. On the other hand, the mass fraction of π-Al8FeMg3Si6 phase increased significantly. The compact π-Al8FeMg3Si6 phase exhibited greater resistance to indentation, worked as a barrier to dislocation motion, and was thermally stable at elevated temperatures.
(2) Based on the prediction by the thermodynamic simulation software, the modified Al alloy with the composition of Al-5Si-0.25Cu- 1.2Mg-0.8Mn-0.7Fe-0.25Zn (wt.%) was the most suitable for thixoforming. The uniform distribution of α(Al) solid spheroids with hardly any micro pore was successfully produced after the modified Al alloy was thixoformed. This thixoformed alloy showed increased YS, UTS and microhardness, after being subjected to T6 heat treatment. These improvements in the mechanical properties of the thixoformed-modified Al alloy after T6 were due to the morphological change of eutectic Si structure from fibrous to spheroidal shape and the precipitation hardening of the very fine Mg2Si particles. Treating the modified Al alloy at the high solid solution temperature of 525 °C encouraged Mg2Si dissolution. This phenomenon led to a high diffusion rate of Mg in Al, which helped complete the aging process.
Acknowledgements
The authors gratefully acknowledge the National University of Malaysia (Universiti Kebangsaan Malaysia, UKM) and the Ministry of Education (MOE) of Malaysia for the financial support received under research grant DIP-2016-007.
References
[1] OMAR M Z, PALMIERE E J, HOWE A A, ATKINSON H V, KAPRANOS P. Thixoforming of a high performance HP9/4/30 steel [J]. Materials Science and Engineering A, 2005, 395: 53-61. https://doi.org/10.1016/j.msea.2004.12. 013.
[2] ARIF M A M, OMAR M Z, MUHAMAD N. Effect of solid solution treatment on semisolid microstructure of Zn-22Al alloy [J]. Pertanika Journal of Science and Technology, 2012, 20(1): 121-127.
[3] ARIF M A M, OMAR M Z, MUHAMAD N, SYARIF J, KAPRANOS P. Microstructural evolution of solid-solution- treated Zn-22Al in the semisolid state [J]. Journal of Materials Science and Technology, 2013, 29: 765-774. https://doi.org/10.1016/j.jmst.2013.04.003.
[4] HIRT G, DREMER R, WITULSKI T, TINIUS H C. Lightweight near net shape components produced by thixoforming [J]. Materials and Design, 1997, 18: 315-321. https://doi.org/10.1016/S0261-3069(97)00071-X.
[5] CHEN T J, HAO Y, SUN J. Microstructural evolution of previously deformed ZA27 alloy during partial remelting [J]. Materials Science and Engineering A, 2002, 337: 73-81. https://doi.org/10.1016/S0921-5093(02)00018-7.
[6] KIRKWOOD D H. Semisolid metal processing [J]. International Materials Reviews, 1994, 39: 173-189. https:// doi.org/10.1179/imr.1994.39.5.173.
[7] OMAR M Z, ATKINSON H V, KAPRANOS P. Semi-solid metal processing-A processing method under low flow loads [J]. Jurnal Kejuruteraan, 2007, 19: 137-146.
[8] OMAR M Z, ATKINSON H V, HOWE A A, PALMIERE E J, KAPRANOS P, GHAZALI M J. Solid-liquid structural break-up in M2 tool steel for semi-solid metal processing [J]. Journal of Materials Science, 2009, 44: 869-874. https://doi. org/10.1007/s10853-008-3181-1.
[9] ARIF M A M, OMAR M Z, SAJURI Z, MOHAMED I F, SAMAT S. The effects of cooling slope on the semi-solid microstructures of Al4.8Si2.8Cu0.5Mg aluminium alloy [J]. Journal of Mechanical Engineering, 2018, 7(Special Issue): s231-s239.
[10] SALLEH M S, OMAR M Z, SYARIF J, ALHAWARI K S, MOHAMMED M N. Microstructure and mechanical properties of thixoformed A319 aluminium alloy [J]. Materials and Design, 2014, 64: 142-152. https://doi.org/10. 1016/j.matdes.2014.07.014.
[11] CHEN G, ZHOU T, WANG B, LIU H, HAN F. Microstructure evolution and segregation behavior of thixoformed Al-Cu-Mg-Mn alloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 39-50. https://doi.org/10.1016/S1003-6326(16)64086-4.
[12] ATKINSON H V, RASSILI A. A review of the semi-solid processing of steel [J]. International Journal of Material Forming, 2010, 3(1): 791-795. https://doi.org/10.1007/ s12289-010-0889-7.
[13] HAGA T, NAKAMURA R, TAGO R, WATARI H. Effects of casting factors of cooling slope on semisolid condition [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S): s968-s972. https://doi.org/10.1016/S1003-6326(10) 60615-2.
[14] BIROL Y. A357 thixoforming feedstock produced by cooling slope casting [J]. Journal of Materials Processing Technology, 2007, 186: 94-101. https://doi.org/10.1016/j.jmatprotec.2006. 12.021.
[15] ALHAWARI K S, OMAR M Z, GHAZALI M J, SALLEH M S, MOHAMMED M N. Evaluation of the microstructure and dry sliding wear behaviour of thixoformed A319 aluminium alloy [J]. Materials and Design, 2015, 76: 169-180. https://doi.org/10.1016/j.matdes.2015.03.057.
[16] PAES M, ZOQUI E J. Semi-solid behaviour of new Al-Si-Mg alloys for thixoforming [J]. Materials Science and Engineering A, 2005, 406: 63-73. https://doi.org/10.1016/j. msea. 2005.07.018.
[17] ARIF M A M, OMAR M Z, SAJURI Z. Formation of spheroidal microstructure in semi-solid state of Al-4.8Si- 2.8Cu-0.5Mg aluminium alloy [J]. Jurnal Kejuruteraan, 2018, 30(2): 275-280. http://dx.doi.org/10.17576/jkukm- 2018-x30(2)-18.
[18] OTHMAN K, GHANI J A, HARON C H C, JURI A, KASSIM M S. Microstructural study of aluminium alloy A390 after milling process [J]. Jurnal Kejuruteraan, 2018, 30(2): 257-264. http://dx.doi.org/10.17576/jkukm-2018- 30(2)-16.
[19] LIU D, ATKINSON H V, JONES H. Thermodynamic prediction of thixoformability in alloys based on the Al-Si-Cu and Al-Si-Cu-Mg systems [J]. Acta Materialia, 2005, 53: 3807-3819. https://doi.org/10.1016/j.actamat.2005. 04.028.
[20] MACIEL CAMACHO A, ATKINSON H V, KAPRANOS P, ARGENT B B. Thermodynamic prediction of wrought alloy compositions amenable to semi-solid processing [J]. Acta Materialia, 2003, 51: 2319-2330. https://doi.org/10.1016/ S1359-6454(03)00040-5.
[21] ZOQUI E J, BENATI D M, PRONI C T W, TORRES L V. Thermodynamic evaluation of the thixoformability of Al-Si alloys [J]. Calphad, 2016, 52: 98-109. https://doi.org/10. 1016/j.calphad.2015.12.006.
[22] ASM Handbook. Properties and selection: Nonferrous alloys and special-purpose materials [M]. Vol. 2. United States of America: ASM International, 1990.
[23] SALLEH M S, OMAR M Z, SYARIF J. The effects of Mg addition on the microstructure and mechanical properties of thixoformed Al-5%Si-Cu alloys [J]. Journal of Alloys and Compounds, 2015, 621: 121-130. https://doi.org/10.1016/ j.jallcom.2014.09.152.
[24] SADEGHI I, WELLS M A, ESMAEILI S. Modeling homogenization behaviour of Al-Si-Cu-Mg aluminium alloy [J]. Materials and Design, 2017, 128: 241-249. https:// doi.org/10.1016/j.matdes.2017.05.006.
[25] MONDOLFO L F. Aluminium alloys: Structure and properties [M]. London: Butterworths, 1976.
[26] XU X, YANG Z, YE Y, WANG G, HE X. Effects of various Mg/Si ratios on microstructure and performance property of Al-Mg-Si alloy cables [J]. Materials Characterization, 2016, 119: 114-119. https://doi.org/10.1016/j.matchar.2016.07.011.
[27] ABEDI A, SHAHMIRI M, AMIR ESGANDARI B, NAMI B. Microstructural evolution during partial remelting of Al-Si alloys containing different amounts of magnesium [J]. Journal of Materials Science and Technology, 2013, 29(10): 971-978. https://doi.org/10.1016/j.jmst.2013.04.021.
[28] SAMUEL A M, OUELLET P, SAMUEL F H, DOTY H W. Microstructural interpretation of thermal analysis of commercial 319 Al alloy with Mg and Sr additions [J]. Transactions of the American Foundrymen’s Society, 1998, 105: 951-962.
[29] SJOLANDER E, SEIFEDDINE S. The heat treatment of Al-Si-Cu-Mg casting alloys [J]. Journal of Materials Processing Technology, 2010, 210: 1249-1259. https:// doi.org/10.1016/j.jmatprotec.2010.03.020.
[30] TAYLOR J A, ST JOHN D H, BARRESI J, COUPER M J. Influence of Mg content on the microstructure and solid solution chemistry of Al-7%Si-Mg casting alloys during solution treatment [J]. Materials Science Forum, 2000, 331-337: 277-282. https://doi.org/10.4028/www.scientific. net/MSF.331-337.277.
[31] ALHAWARI K S, OMAR M Z, GHAZALI M J, SALLEH M S, MOHAMMED M N. Microstructural evolution during semisolid processing of Al-Si-Cu alloy with different Mg contents [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 1483-1497. https://doi.org/10.1016/S1003- 6326(17)60169-9.
[32] MOUSTAFA M A, SAMUEL F H, DOTY H W. Effect of solution heat treatment and additives on the microstructure of Al-Si (A413.1) automotive alloys [J]. Journal of Materials Science, 2003, 38: 4507-4522. https://doi.org/10.1023/A: 1027333602276.
[33] AMIN-AHMADI B, AASHURI H. Semisolid structure for M2 high speed steel prepared by cooling slope [J]. Journal of Materials Processing Technology, 2010, 210: 1632-1635. https://doi.org/10.1016/j.jmatprotec.2010.05.011.
[34] KOUTSOUKIS T, MAKHLOUF M M. An alternative eutectic system for casting aluminum alloys II. Modification of the eutectic morphology [M]// HYLAND M. Light Metals. Springer, Cham, 2015. https://doi.org/10.1007/978-3-319- 48248-4_48.
[35] MANENTE A, TIMELLI G. Optimizing the heat treatment process of cast aluminium alloys: Recent trends in processing and degradation of aluminium alloys [M]// IntechOpen, 2011. https://www.intechopen.com/books/ recent-trends-in-processing-and-degradation-of-aluminium-alloys/optimizing-the-heat-treatment-process-of-cast-aluminium-alloys.
[36] LIU S, LI Q, LIN H, SUN L, LONG T, YE L, DENG Y. Effect of quench-induced precipitation on microstructure and mechanical properties of 7085 aluminum alloy [J]. Materials and Design, 2017, 132: 119-128. https://doi.org/10.1016/j. matdes.2017.06.054.
[37] TAVITAS-MEDRANO F J, GRUZLESKI J E, SAMUEL F H, VALTIERRA S, DOTY H W. Effect of Mg and Sr-modification on the mechanical properties of 319-type aluminium cast alloys subjected to artificial aging [J]. Materials Science and Engineering A, 2008, 480: 356-364. https://doi.org/10.1016/j.msea.2007.09.002.
M. A. M. ARIF1, M. Z. OMAR1, Z. SAJURI1, M. S. SALLEH2
1. Centre for Materials Engineering and Smart Manufacturing (MERCU), Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600, Selangor, Malaysia;
2. Department of Manufacturing Process, Faculty of Manufacturing Engineering, Universiti Teknikal Melaka, Hang Tuah Jaya, 76100 Durian Tunggal, Melaka, Malaysia
摘 要:触变成形是使金属在半固态时发生变形的一种加工方法。该工艺的优点包括具有良好的表面光洁度,细小的显微组织和优越的力学性能,然而,该工艺主要是用铝铸件生产零件,没有充分利用其真正潜力。因此,可用热力学模拟进行合金成分设计。研究减少铜含量和增加硅、镁含量对2014铝合金触变成形的影响,包括模拟和实验验证两部分。结果表明,增加合金中硅含量和降低铜含量,可以降低合金的凝固区间温度,扩大特定液相分数之间的工作温度窗口,这对该工艺有利。高的固溶温度导致Mg2Si化合物的分解。Mg含量的增加导致致密π-Al8FeMg3Si6相的形成和尖片状结构β-Al5FeSi相的减少,提高合金的强度。后续T6热处理进一步提高改性合金的强度。
关键词:JMatPro;锻造2014铝合金; 触变成形;半固态加工;力学性能
(Edited by Bing YANG)
Corresponding author: M. A. M. ARIF, M. Z. OMAR; Tel: +60-3-8911-8010; Fax: +60-3-8925-2546; E-mail: anifarif@gmail.com,zaidiomar@ukm.edu.my
DOI: 10.1016/S1003-6326(20)65212-8

