
Microcalorimetric studies of interaction between extracellular polymeric substance and sulfide minerals
ZHU Jian-yu(朱建裕)1, 2, YANG Peng(杨 鹏)1, LI Bang-mei(李邦梅)1,
ZHANG Jing-xia(张静霞)1, HUANG Qiu-xia(黄秋霞)1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy of Ministry of Education, Central South University, Changsha 410083, China
Received 20 September 2008; accepted 5 November 2008
Abstract: Extracellular polymeric substance(EPS) seem to be important for bioleaching. For better control of the processes, the function of EPS of leaching bacteria is of crucial importance. Microcalorimetric measurement was used to study the three simulative components of EPS. The attachment of L-cysteine increases with its increasing concentration, which is Langmuir monomolecular layer adsorption. The attachment of glucose on pyrite is similar to mannose, which is not Langmuir monomolecular layer but multilayer adsorption. The results prove that the EPS mediates attachment and reacts with the sulphide surface and results in changed chemical properties of the mineral surface. It provides a new method to study the interaction between EPS and sulfide minerals.
Key words: extracellular polymeric substance; sulfide mineral; pyrite; microcalorimetry; attachment
1 Introduction
It is increasingly accepted that extracellular polymeric substance(EPS) plays a pivotal role in biocorrosion of metal sulfides for the winning of precious metals[1]. In bioleaching, bacteria attach on substrates and form biofilms that have well-developed community structures, with mechanisms for the delivery of nutrients and the disposal of waste products[2]. The EPS is thought to mediate attachment. Cells with the EPS layer removed cannot attach covellite until the EPS layer is regenerated[3]. The iron(Ⅲ) ions in the EPS give the cell surface a net positive charge under physiological conditions (pH=2), where pyrite is negatively charged[4]. Thus, the EPS complexed with iron(Ⅲ) ions enables the cells to interact with pyrite surface through electrostatic forces. They also play an active role in the dissolution of pyrite via an indirect mechanism. The EPS containing complexed iron(Ⅲ) ions comprises a reaction space, in which the dissolution process takes place[5-6]. The EPS might facilitate the concentration of iron(Ⅲ) by complexation through uronic acids or other residues on the mineral surface, resulting in an enhanced oxidative attack on the sulphide [7]. Microbial attachment and EPS formation may provide a mechanism through which the microorganism can locate itself near an energy source.
Microcalorimetry is a useful tool that has been increasingly employed in medical and biological areas. It allows the study of biology at the molecular level as well as at the cellular level[8-9]. The method has the advantage of being specific only to the initial and final energy states of a system and it is independent of organisms and reaction pathway. The calorimetry can directly determine the biological activity of a living system and provide a continuous measurement of heat production, which gives much information in both qualitative and quantitative ways[10].
Until now, there are no reports about the study of interaction mechanism between EPS and pyrite by means of thermodynamics. In contrast to bacteria grown in different substrates, the content of glucose is the highest and the mannose content is the minimum[11]. It is reported that cysteine can accelerate the bioleaching remarkably[12]. The composition of the EPS deserves considerable attention in order to optimize bioleaching processes or to inhibit acid rock drainage[7]. In this work, the microcalorimetric measurement was used to study the interaction between the three simulative components of EPS and the surface of pyrite, and the pivotal role of EPS in bioleaching was demonstrated.
2 Experimental
2.1 Materials
Pyrite used in this experiment was well-crystallized mineral from geological museum of Hunan Province, China. After ultrasonic treatment, in order to remove the surface oxide resulting from exposure to air, mineral particles were well ground in agate mortar until the mesh size was smaller than 2 μm. The powder was sealed and reserved in a jar.
L-cysteine, D-glucose and D-mannose were all biochemica1 reagents. Water used in the experiment was double distilled.
2.2 Equipment
The heat-flow microcalorimeter, thermometric 3114/3236 TAM Air eight-channel calorimeter (Thermometric AB, Sweden) was a thermal activity monitor controlled by Picolog software. Each calorimetric channel was constructed in twin configuration with one side for the sample and the other side for a static reference. The microreaction system was a titration mode with a 20 mL ampoule. Continuous heat leakage measurements were taken in an isothermal system. The heat of adsorption flowed through high-sensitivity thermopiles surrounded by a heat sink, which was stabilized at ±2×10-4. The magnitude of heat exchange of thermopile with a heat sink was proportional to the time interval of the voltage signal. The electrical calibration performance allowed us to quantify the results.
2.3 Methods
The experiment was done with different mass ratios of mineral to reagent and different time. The temperature was unvarying at 30 ℃. The final volume of the reaction system was 10 mL. The concentration of pyrite was 0.03 g/mL. After the baseline was stabilized, the reagents of different concentration were pumped into the ampoules. When the reaction finished, the power—time curve was obtained using Picolog software.
3 Results and discussion
3.1 Power—time curves produced by L-cysteine and pyrite
The mass ratios of mineral to L-cysteine (mp/mc) were 0.8, 4, 8, 16, 40, 60 and 80, and the time of reaction was 100 min.
From analysis of the power—time curves in Fig.1(a), it can be seen that the maximum power heights appear after 10 min, and the heat given out is the greatest. It accords with the results of WANG et al[13] that after about 15 min the L-cysteine reaches the maximum attachment. The best mass ratio of pyrite to L-cysteine is 4, at which the maximum heat releases. The larger the mass ratio of pyrite to L-cysteine, the lower the concentration of L-cysteine, and the smaller the total heat releases.

Fig.1 Power—time curves for attachment of L-cysteine on pyrite with different mp/mc (a) and relationship between different mp/mc and integral heat (Qt) (b)
Fig.1(b) shows that when the attachment is maximum, the concentration of L-cysteine is about 7.5× 10-3 g/mL. A saturation of heat of reaction in the process of L-cysteine attachment on pyrite occurs with the increase of the concentration of L-cysteine. L-cysteine concentration increases to a certain extent, and the attachment is in the balance. In the low concentration of solution, the adsorption is very similar to the gas adsorbing to the solid. Thereby, it is reasonable to use Langmuir adsorption model and BET theory to elucidate the monomolecular layer attachment and multilayer physical attachment, respectively. It can be inferred that it is analogous to Langmuir monomolecular layer attachment and chemical attachment occurred between L-cysteine and pyrite surface. Pyrite is negatively charged, and it probably results in the interaction with the NH3+ of cysteine’s zwitterion because of the electrostatic forces. It is conducive to the attachment. Cysteine is the main anion form when the pH is high. It is mutually exclusive with the surface of pyrite and not easy for bacteria attaching to the pyrite. It can explain why the solution pH is controlled to about 1.8 in bioleaching. It is not only to optimize bacterial activity, but also is hard for bacteria to attach the surface of mineral if the pH is too high. In addition, the sulpho-hydryl groups of L-cysteine might react with the sulphide surface with the subsequent release of iron and sulphur species. Bacteria can take advantage of the released species that are in favor of the biochemical corrosion process[14]. The EPS maybe result in changed properties of the mineral surface[15].
3.2 Power—time curves produced by glucose and pyrite
The mass ratios of mineral to glucose (mp/mg) are 0.8, 1.0, 2.0, 4.0, 8.0, 40 and 80, and the reaction time is 80 min.
Fig.2(a) shows that in a very short time, the heat releases and the maximum heat peak appears quickly. The attachment of glucose on pyrite appears as a fast procedure. The optimal mass ratio is 0.8. At this point the largest energy appears. The same as L-cysteine, with the increase of the concentration of glucose, the heat released increases. It can be inferred that the interaction between EPS and sulfide minerals is rapid.
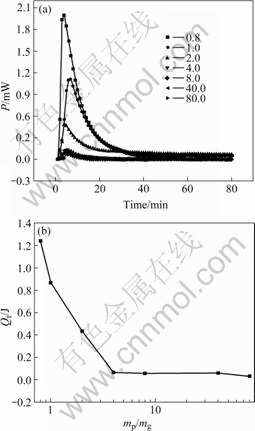
Fig.2 Power—time curves for attachment of glucose on pyrite with different mp/mc (a) and relationship between different mp/mc and integral heat (Qt) (b)
From Fig.2(b), the heat increases quickly after the concentration of glucose reaches 7.5×10-3 g/mL and there is no attachment balance appearing. The heat of reaction increases constantly with the increase of the glucose, which is not Langmuir monomolecular layer but BET multilayer attachment[16]. It is physical attachment. There is van der Waals force between glucose and the surface of pyrite. It urges the glucose to form monomolecular layer on the pyrite. Because of the van der Waals force between the glucose molecules, the glucose ulteriorly adsorbs the primary monomolecular layer. Multilayer is formed after the continuous adsorption. BET theory hypothesizes that only the solute of the first layer contacts with the surface of the solid. The heat given out of the first layer is the largest, which is approximately equal to the chemical reaction. The attachment of the other layers relies on the van der Waals force. Thus, the heat of each layer is nearly equivalent. Fig.2(a) accords with the hypothesis. All baselines are reposeful after a transitory heat flood tide. That is, the monomolecular layer of glucose attaches to the surface of pyrite, so as to the latter glucose molecules cumulate ceaselessly.
3.3 Power—time curves produced by mannose and pyrite
The mass ratios of mineral to mannose (mp/mm) are 1.0, 1.7, 2.0, 2.5 and 4.0, and the reaction time is 60 min (Fig.3).
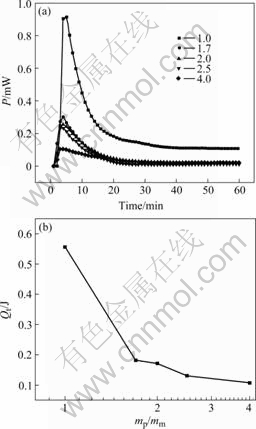
Fig.3 Power—time curves for attachment of mannose on pyrite with different mp/mm (a) and relationship between different mp/mc and integral heat (Qt) (b)
As similar to glucose, the heat given out increased quickly. This further proves that attachment is a rapid process. The heat of reaction is enhanced as the concentration of mannose increases to more than 1.7× 10-3 g/mL, which is lower than glucose. It may be by the reason of the different relative molecular mass. The final baselines are balanced with different mannose concentrations. It is also multilayer physical attachment between mannose and pyrite. Langmuir adsorption model and BET theory are well to illustrate the power—time curves of L-cysteine, glucose and mannose respectively. However, the two theories hypothesize that the surface of solid is symmetrical. In practice, the mineral surface and the interaction between molecules can affect the attachment.
The EPS containing complexed Fe3+ is the relevant agent in the dissolution process of pyrite. Fe3+ reduction by attached bacteria could be a relevant process involving the EPS layer in the bioleaching of sulphidesin aerobic conditions[7]. The adsorption of glucose and mannose might facilitate the concentration of Fe3+ by complexation through uronic acids or other residues on the mineral surface[17], resulting in an enhanced oxidative attack on the sulphide.
4 Conclusions
1) The results prove the function of EPS. It provides a new method, microcalorimetry, to study the interaction between EPS and sulfide minerals. This method excels the conventional measures because it is quick and sensitive, and has slight effect on the sample.
2) The attachment of L-cysteine is Langmuir monomolecular layer adsorption. L-cysteine might react with the sulphide surface and lead to change in the characteristic of the surface chemistry. The chemical reactions result in corrosion of the mineral surface and release some substances which the bacteria can utilize.
3) The attachments of glucose and mannose show that the attachment is rapid and specific. They are multilayer attachments and only the physical reaction has taken place. Because of the continuous increase of the adsorption, glucose and mannose might facilitate the concentration of Fe3+ by complexation through uronic acids or other residues on the mineral surface, resulting in an enhanced oxidative attack on the sulphide, so as to promote the bioleaching.
References
[1] WOLFGANG S, TILMAN G. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria [J]. Research in Microbiology, 2006, 157: 49-56.
[2] WATLING H R. The Bioleaching of sulphide minerals with emphasis on copper sulphide—A review [J].Hydrometallurgy, 2006, 84: 81-108.
[3] POGLIANI C, DONATI E. The role of exopolymers in the bioleaching of a non-ferrous metal sulphide [J]. Journal of Industrial Microbiology and Biotechnology, 1999, 22: 88-92.
[4] BLAKE II R C, LYLES M M, SIMMONS R C. Morphological and physical aspects of attachment of Thiobacillus ferrrooxidans [J]. Biohydrometallurgical Processing, 1995, 30: 13-22.
[5] TRIBUTSCH H. Direct versus indirect bioleaching [J]. Hydrometallurgy, 2000, 59: 177-185.
[6] FOWLER T A, HOLMES P R, CRUNDWELL F K. Mechanism of pyrite dissolution in the presence of Thiobacillus ferrooxidans [J]. Applied and Environmental Microbiology, 1999, 65(7): 2987-2993.
[7] KINALER K, GEHRKE T, TELEGDI J, SAND W. Bioleaching—A result of interfacial processes caused by extracellular polymeric substances (EPS) [J]. Hydrometallurgy, 2003, 71: 83-88.
[8] JIANG D, HUANG Q, CAI P, RONG X, CHEN W. Adsorption of Pseudomonas putida on clay minerals and iron oxide [J]. Colloids and Surfaces B: Biointerfaces, 2007, 54: 217-221.
[9] CAI Peng, HUANG Qiao-yun, JIANG Dai-hua, RONG Xing-min, LIANG Wei. Microcalorimetric studies on the adsorption of DNA by soil colloidal particles [J]. Colloids and Surfaces B: Biointerfaces, 2006, 49: 49-54.
[10] XU Xiang-jiao, XUE Zhi, QI Zu-de, HOU An-xin, LI Chao-hong, LIU Yi. Antibacterial activities of manganese(II) ebselen-porphyrin conjugate and its free components on Staphylococcus aureus investigated by microcalorimetry [J]. Thermochimica Acta, 2008, 476: 33-38.
[11] HARNEIT K, GOKSEL A, KOCK D, KLOCK J H, GEHRKE T, SAND W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans [J]. Hydrometallurgy, 2006, 83: 245-254.
[12] ROJAS-ChAPANA J A, TRIBUTSCH H. Bio-leaching of pyrite accelerated by cysteine [J]. Process Biochemistry, 2000, 35: 815-824.
[13] LIU Jian-she, WANG Zhao-hui, LI Bang-mei, ZHANG Yan-hua. Interaction between pyrite and cysteine [J]. Trans Nonferrous Met Soc China, 2006, 16(4): 943-946.
[14] TRIBUTSCH H, ROJAS-ChAPANA J A. Metal sulfide semiconductor electrochemical mechanisms induced by bacterial activity [J]. Electrochimica Acta, 2000, 45: 4705-4716.
[15] LIZAMA H M, FAIRWEATHER M J, DAI Z, ALLEGRETTO T D. How does bioleaching start? [J]. Hydrometallurgy, 2003, 69: 109-116.
[16] MENG M, LORENZO S, JEAN-FRANOIS L. Adsorption and thermal condensation of glycine on SiO2 [J]. Chinese Journal of Catalysis, 2005, 26: 393-398. (in Chinese)
[17] LI X Y, YANG S F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge [J]. Watere Research, 2007, 41: 1022-1030.
Foundation item: Project(2004CB619201) supported by the National Basic Research Program of China; Project(50621063) supported by the National Natural Science Foundation of China
Corresponding authors: ZHU Jian-yu; Tel: +86-731-8836944; E-mail: csuzhu@yahoo.com.cn
(Edited by LI Xiang-qun)