Electronic structure of Au-Cu alloys
YU Fang-xin(余方新), XIE You-qing(谢佑卿), NIE Yao-zhuang(聂耀庄),
LI Xiao-bo(李小波), PENG Hong-jian(彭红键), TAO Hui-jin(陶辉锦)
(School of Materials Science and Engineering, Central South University, Changsha 410083, China)
Abstract: By studying the correlativity between energy, volume and electronic structure of characteristic crystals and bound conditions of OA theory, the potential energy function, atomic volume interactive function and electronic structure of Au-Cu alloys have been determined. Then following the general Vegards law in characteristic theory, the electronic structure and properties of disordered continue solid solution and three ordered alloys with the maximum ordering degree are calculated. It is found that the non-bonding electrons and near-free electrons in outer shell will transform to covalent electrons during alloying. By analyzing the variation of electronic structure and cohesion of ordering and disordered alloys, the transformation of order-disorder transition Au-Cu alloy has been studied.
Key words: CC theory; electronic structure; Au-Cu alloy; ordering transformation CLC number: TG111
Document code: A
1 INTRODUCTION
As a traditional alloy, Au-Cu system is extensively applied in catalysis, electronic industry and biological material field. Recently, some new functions of the alloy are found in nano-crystal and thin film materials[1-4]. It is well known that there exist transformations of order-disorder transition in Au-Cu system. Comprehensive theoretical and experimental studies have been contributed to these transformations[5-8]. Most of these studies are focused on the thermodynamic properties and dynamic process. The present paper attempts to explore the intrinsic causes of order-disorder transitions by studying the interactions of the constituents and the correlativity among energy, volume and electronic structure of characteristic crystals in the alloy.
The potential energy and volume interaction functions of Au-Cu alloy are established according to CC theory, and then the electronic structures and physic properties of ordering and disordering structures in the alloy are calculated. As a consequence the intrinsic causes of order-disorder transformation in the alloy are analyzed.
2 INTERACTION FUNCTIONS OF POTENTIAL ENERGY AND VOLUME IN DISORDERED ALLOY
Characteristic crystals(CC) theory[9-14]are developed from central atoms model[15]. According to the central atoms model, even atoms of the same component in solid solution alloys may have different electronic structures and properties due to variant environment, i.e. variant coordination number and coordinate atoms, and separate into different characteristic atoms. For alloys of two components, there are characteristic atoms: A0…Ai…AI, B0…Bi…BI where i is the number of B atoms in the nearest neighboring shell, and I is the coordination number. Characteristic crystal consists of one certain characteristic atom and has the same crystal structure with the alloy. Ref.[9] derived the relationship between i and atoms potential energy and volume of characteristic crystals. Table 1 lists the interaction functions of potential energy and volume in disordered alloy.
3 POTENTIAL ENERGY FUNCTIONS OF Au-Cu ALLOYS
The parameters and EAu0 and ECu0are, respectively, molar potential energies of characteristic crystal with primary states. Because the Au-Cu alloys have the same type of fcc crystal structure as that of pure elements Au and Cu, the characteristic crystals with primary states ψAu0 and ψCuI are respectively pure elements with atomic states ψ0Au and ψ0Cu. From this, it can be known that EAu0=E0Au, ECuI=ECu where EAu and ECu are equal to negative value of cohesive energies of pure metal Au and Cu respectively. The parameters EAuI and ECu0 are, respectively, molar potential energies of characteristic crystal with terminal states ψAuI and ψCu0. The values of EAuI and ECu0 in the nine potential interaction functions can be, in principle, determined by experimental values of heat of formation of only two alloys.
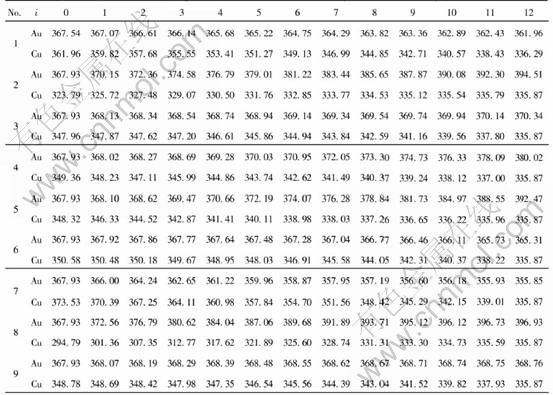
Once the values of parameters EAu0, EAuI, ECu0 and ECuI are known, the cohesive energies of characteristic crystal can be calculated from the corresponding potential functions of components where the subscript i is the number of Cu atoms in the nearest neighboring configuration and can change from 0 to I. I is the coordination number and is equal to 12 for Au-Cu alloys. Table 2 lists the cohesive energies of Au and Cu characteristic in Au-Cu alloys.
Table 1 Interaction functions of potential energy(Q=E) and volume(Q=V) of disordered binary solid solution
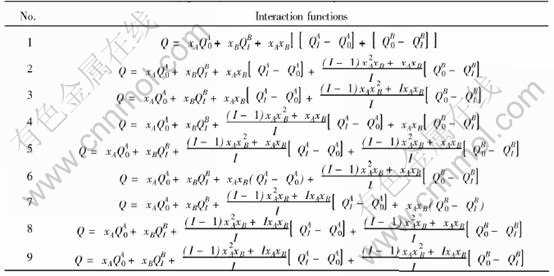
Table 2 Cohesive energies of Au and Cu characteristic crystals in Au-Cu alloys(kJ/mol)
4 VOLUME INTERACTION FUNCTIONS OF Au-Cu ALLOYS
If V is used instead of E, nine potential energy functions in Table 1 become nine V-functions of CC theory. Parameters VAu0 and VCuI are respectively the atom volumes with primary state of Au and Cu characteristic crystals. Because the Au-Cu alloys have the same type of fcc crystal structure as that of pure elements Au and Cu, the characteristic crystals with primary states ψAu0 and ψCuI are respectively pure elements with atomic states ψ0Au and ψ0Cu. From this it can be known that, VAu0=V0Au, VCuI=V0Cu. The parameters, VAuI and VCu0, are respectively atomic volumes of terminal characteristic crystals ψAuI and ψCu0 of components Au and Cu. The values of the nine V-function can be, in principle, determined by experimental values of lattice constants of only two alloys.
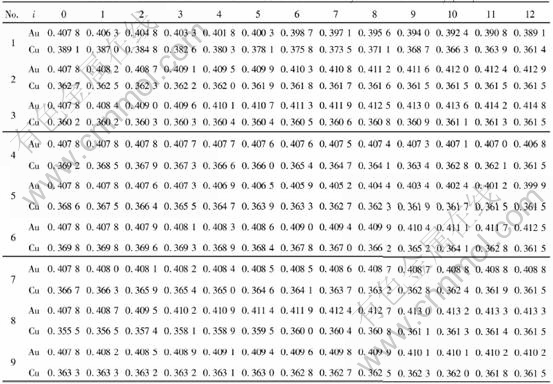
Once the values of VAu0, VAuI, VCuI and VCu0 of each V-function are known, the lattice constants aAui and VCui of characteristic crystal, obtained by equation(a=(4V)1/3), can be calculated from the atomic volume equations of components corresponding to each V-function of the Au-Cu alloys. The results are listed in Table 3.
5 ELECTRONIC STRUCTURE OF CHARACTERISTIC CRYSTAL IN Au-Cu ALLOYS
Obtaining the cohesive energies and lattice constants of characteristic crystals in alloys, the electronic structure can be calculated according to OA theory[16, 17]. In OA theory, the electronic structures of pure metals is described by the quasi-electron-occupation(QEO) number of the one-atom state ψa which is made up of some basic atom states φk:

The atoms are condensed into the crystal by metallic bond, which is a mixing bond of un-saturated covalent electronic bond, free electronic bond and magnetic electronic bond. Therefore, there exist covalent electrons(nc), near-free electrons(nf), magnetic electrons(nm), and non-bond electrons(nn) in the metals. The potential function with many-atom interactions in solid relates the atomic state to the cohesive energy and lattice constant of the crystal. For a given crystal with a certain electronic structure, a set of more complete data about cohesive energy, lattice constants, bulk modulus, elastic modulus, shear
Table 3 Lattice constants of Au and Cu characteristic crystals in Au-Cu alloys(nm)
modulus, Poissons ratio, specific heat and thermal expansion coefficient as a function of temperature can be obtained. The electronic structure and properties of Au and Cu characteristic crystals in Au-Cu alloys obtained by OA theory are listed in Tables 4 and 5, in which sc and sf refer respectively to covalent electrons and near-free electrons in the s orbit; dc and dn, refer covalent electrons and non-bond electrons in the d orbit, respectively; Ec, cohesive energy(kJ/mol); a, lattice constant equal to (4V)1/3, nm; B, bulk modulus, 1011N·m-2; α, linear thermal expansion coefficient at 293K, 10-6. Some understanding can be obtained from these results.
Electronic structure, cohesive energy, lattice constant and physical properties of each characteristic crystal are correlative. Therefore, the E-function and V-function for a given alloy system must be correlative.
Comparing cohesive energies of Au and Cu characteristic crystals in Tables 4 and 5 with those in Table 2, it can be seen that the correct E-function of Au-Cu alloys is the 5th function in Table 1, namely
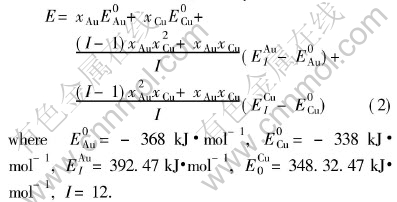
Comparing lattice constants of Au and Cu characteristic crystals in Tables 4 and 5 with those in Table 3, it can be known that the correct V-function of Au-Cu system is the 5th function in Table 1, namely
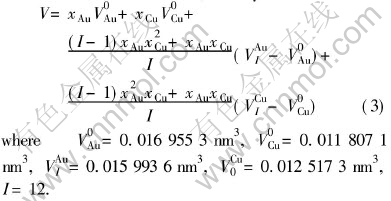
As to Au(Cu) characteristic atoms, atomic potential energies rise as the numbers of Cu(Au) atoms in the nearest neighboring shell increase, which are essentially due to the increase of dc covalent electrons in the Au(Cu) characteristic atoms with increase in the i value. Such changes lead to the tendency to form ordering structure for Au-Cu alloys.
6 ELECTRONIC STRUCTURE AND PROPERTIES OF DISORDERED Au-Cu ALLOYS
According to CC theory, no matter what imaginable variation the atomic arrangement in the
Table 4 Electronic structure and properties of Au characteristic crystals in Au-Cu alloys
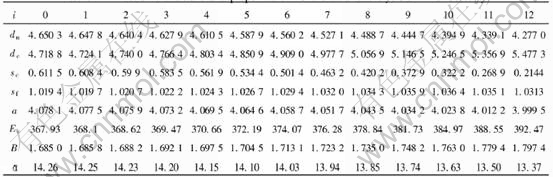
Table 5 Electronic structure and properties of Cu characteristic crystals in Au-Cu alloys
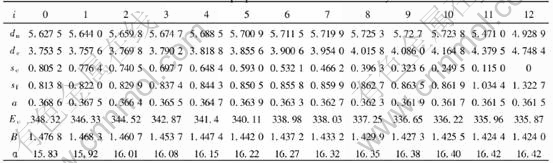 lattice occurs, the relative concentrations of characteristic crystals would change, but their electronic structure and properties of characteristic crystals will not. Therefore, once the electronic structure and properties of characteristic crystals have been determined, no matter what the designed atomic arrangement in the lattice is, the electronic structure and properties of the alloy can be predicted. For disordered alloys, the concentration of characteristic crystals can be calculated by Eqn.(2). Substituting the electronic structure, cohesive energies and atomic volumes of characteristic crystals in Tables 4 and 5 into Eqn.(1), the average electronic structure, cohesive energies and lattice constants of disordered Au-Cu alloys and their components can be obtained.
lattice occurs, the relative concentrations of characteristic crystals would change, but their electronic structure and properties of characteristic crystals will not. Therefore, once the electronic structure and properties of characteristic crystals have been determined, no matter what the designed atomic arrangement in the lattice is, the electronic structure and properties of the alloy can be predicted. For disordered alloys, the concentration of characteristic crystals can be calculated by Eqn.(2). Substituting the electronic structure, cohesive energies and atomic volumes of characteristic crystals in Tables 4 and 5 into Eqn.(1), the average electronic structure, cohesive energies and lattice constants of disordered Au-Cu alloys and their components can be obtained.
The common equations for calculating average electronic structure and properties of components Au and Cu are, respectively
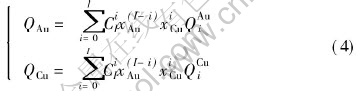
The electronic structure parameters, cohesive energies(kJ/mol) and lattice constants(nm) of disordered Au-Cu alloys and their components are listed in Table 6.
7 ELECTRONIC STRUCTURE AND PROPERTIES OF ORDERED Au-Cu ALLOYS AND INTERMETALLIC COMPOUND
The Au-Cu system is a typic ordering alloy system. According to CC theory, the transformation from a disordered state to ordered state is the degenerative process of characteristic atom. In Au3Cu-L12, there just exist the characteristic atoms 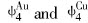 , therefore, the electronic structure of the compound is combined by the characteristic atoms in states
, therefore, the electronic structure of the compound is combined by the characteristic atoms in states 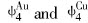 , which is
, which is
0.75[Xe](5dn)4.61(5dc)4.80(6sc)0.56(6sf)1.02+
0.25[Ar](3dn)4.93(3dc)4.75(4sc)0(4sf)1.32
Just the same, the electronic structures of compound AuCu-L10 and Au-Cu3-L12 are
0.5[Xe](5dn)4.49(5dc)5.06(6sc)0.42(6sf)1.03+
0.5[Ar](3dn)5.69(3dc)3.82(4sc)0.64(4sf)0.84
and
0.25[Xe](5dn)4.28(5dc)5.48(6sc)0.21(6sf)1.03+
0.75[Ar](3dn)5.73(3dc)4.02(4sc)0.40(4sf)0.86
respectively.
For the ordered alloys of non-stoichometry, the concentration of the characteristic atoms can be
Table 6 Average electronics and properties of disordered Au-Cu alloys and their components
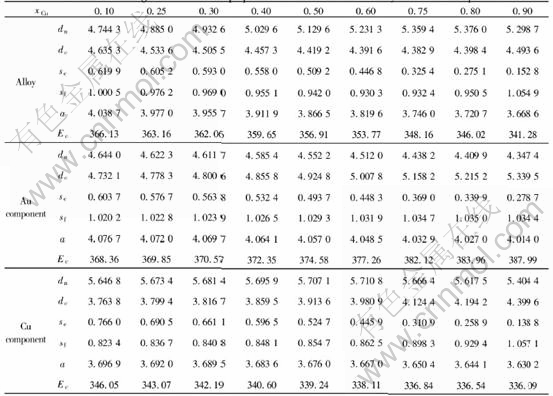
calculated by statistics method. As to fcc AB-L10 type of ordered alloys, the relationship expressions between the concentrations of the characteristic atoms, compositions of the elements and ordering degrees of alloys are
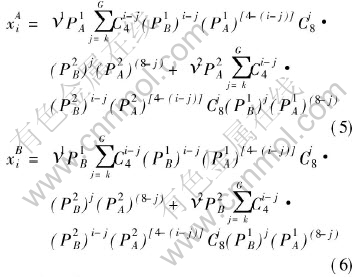
where ν1, ν2 represent the site fraction of sublattice Ⅰ, Ⅱ, respectively, ν1=ν2=1/2; k and G take their values according to

P1A, P1B, P2A, P2B are the probabilities of A and B atom occupying the first and second sublattice, which can be calculated by the equations:
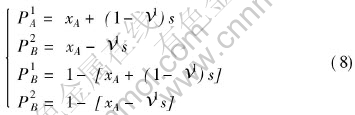
where s is the ordering degree, which is defined as

The maximum ordering degree smax is determined from composition xA of alloys.
For the stoichiometric alloy, due to, P1A=1, smax=1.
For the alloys with xA〈ν1 due to P2A=0, smax=xA/ν1.
For the alloys with xA〈ν1, due to P1A=0, smax=(1-xA)/(1-ν1).
As to fcc A3B-L12 and AB3-L12 type of ordered alloys, there are also equations to calculate the concentrations of characteristic crystals[12].
The average electronic structure and properties of ordered alloys in such types as Au3Cu-L12, AuCu-L10 and AuCu3-L12 with maximum ordering degree as a function of composition are respectively listed in Tables 7, 8 and 9.
Fig.1 illustrates the varying tendencies of
Table 7 Average electronic structure and properties of ordered Au3Cu alloys and their components
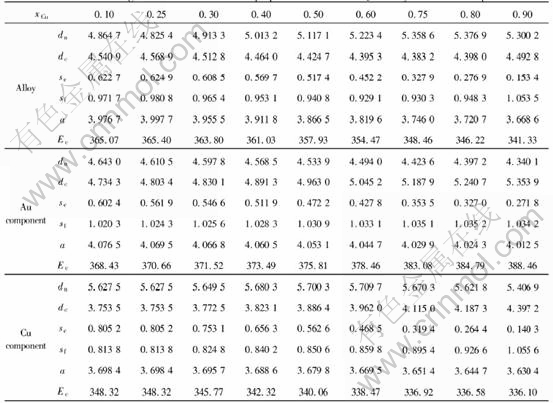
Table 8 Average electronic structure and properties of ordered AuCu alloys and their components
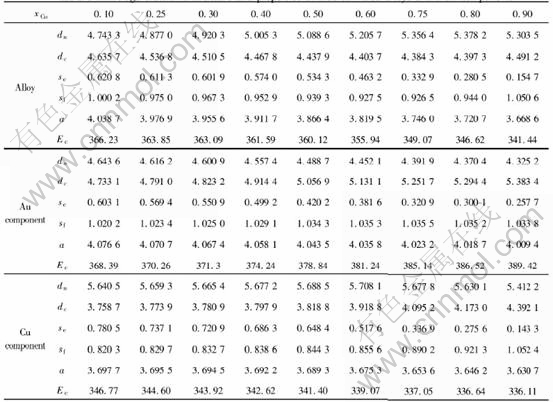
Table 9 Average electronic structure and properties of ordered AuCu3 alloys and their components

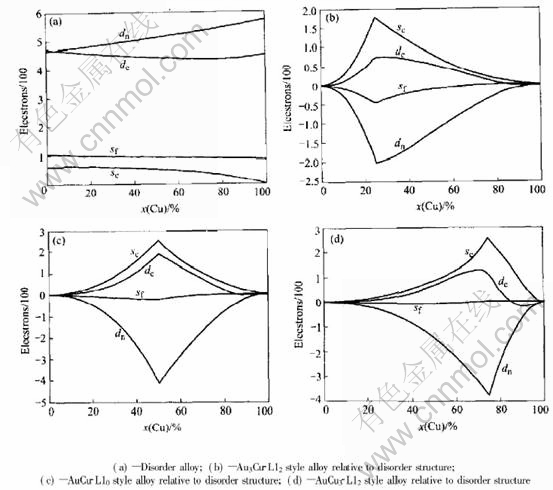
Fig.1 Electronic structure of Au-Cu alloys
electronic structure parameters of Au3Cu-L12, AuCu-L10, AuCu3-L12 type ordered alloys relative to disorder alloy.
8 ANALYZING OF ELECTRONIC STRUCTURE AND ENERGIES DURING TRANSFORMATION BETWEEN ORDERED-DISORDERED ALLOYS
From Fig.1, it can be found that the electronic structure of alloys varies with the transformation of disorder-order transition. Some non-bond electrons and near-free electrons become covalent electrons when the disorder alloys transform to order alloys. At the composition of stoichiometry, there are most electrons to turn to covalent electrons from non-bond and near-free electrons. Its known that the contributions to cohesive energy are different for covalent, non-bond and near-free electron. It can be predicted that there is difference in the cohesive energies of alloys with order and disorder structure. From Tables 7, 8 and 9, it can be seen that, for the alloy of 25%Cu(mole fraction), the cohesive energy of disorder alloy is 363.16kJ/mol, but the cohesive energy of alloy with the structure of Au3Cu-L12 is 365.40kJ/mol; for the alloy of 50%Cu(mole fraction), the cohesive energy of disorder alloy is 356.91kJ/mol, but the cohesive energy of alloy with the structure of AuCu-L10 is 360.12kJ/mol; for the alloy of 75%Cu(mole fraction), the cohesive energy of disorder alloy is 348.16kJ/mol, but the cohesive energy of alloy with the structure of AuCu3-L12 is 351.06kJ/mol. The differences between disorder and order alloy are -2.24, -3.21, and -2.9kJ/mol respectively. This indicates that the order alloys are in lower energy states than disorder alloy, i.e. there exists ordering trend in Au-Cu system.
9 CONCLUSIONS
1) The energy function and volume function of Au-Cu system have been chosen by studying the atomic states, energies and volumes correlatively.
2) The electronic structure, cohesive energies, lattice constants, bulk modulus, and thermal expansion coefficients of characteristic crystals in the Au-Cu system have been determined.
3) The variations of electronic structure, cohesive energies and lattice constants of disordered Au-Cu alloys and three ordering alloys with the maximum ordering degree as a function of composition have been calculated.
4) The transformation of order-disorder transition in the Au-Cu system has been studied based on the analysis of electronic structure and cohesive energies of the order and disorder structures in the system.
REFERENCES
[1]De Goutam, Rao C N R. Two-dimensional Au and Au-Cu alloy nanocrystals with orientation in (111) plane embedded in glassy silica films [J]. Journal of Physical Chemistry B, 2003(11): 13597-13600.
[2]Battezzati L, Baricco M, Belotti M, et al. Microstructure and thermal stability of ‘Nanocrystalline’ electrodeposited Au-Cu alloys [J]. Materials Science Forum,2001, 360: 253-260.
[3]Zhai H R, Zhou S M, Huang H B, et al. Magneto-optical Kerr effect of Fe/Au-Cu and Fe/Al-Cu bilayers [J]. Journal of Magnetism and Magnetic Materials, 1992, 2: 1015-1016.
[4]Sakurai Makoto, Shinjo Teruya. Magneto-optical properties and magnetic anisotropies for Au/Cu/Au/Co and Cu/Au/Cu/Co multilayers [J]. Journal of Applied Physics, 1993, 1: 6840-6846.
[5]Darby S, Mortimer-Jones T V. Theoretical study of Cu-Au nanoalloy clusters using a genetic algorithm [J]. Journal of Chemical Physics, 2002, 22: 1536 -1550.
[6]Han X J, Chen M, Guo Z Y. Thermophysical properties of undercooled liquid Au-Cu alloys from molecular dynamics simulations [J]. Journal of Physics Condensed Matter, 2004, 16(6): 705-713.
[7]Bykov A, Pastukhov E. Calorimetric study of alloy formation in Au-Cu and Au-In systems [J]. Journal of Thermal Analysis and Calorimetry, 2000, 60(3): 845-850.
[8]Sundman B, Fries S G, Oates W A. CALPHAD assessment of the Au-Cu system using the cluster variation method [J]. Zeitschrift fuer Metallkunde/Materials Research and Advanced Techniques, 1999, 90(4): 267-273.
[9]XIE You-qing. Atomic energies and Gibbs energy functions of Ag-Cu alloys [J]. Science in China Ser E, 1998, 41(2): 146-156.
[10]XIE You-qing. Atomic volumes and volume functions for Ag-Cu alloys [J]. Science in China Ser E, 1998, 41(2): 157-168.
[11]XIE You-qing, ZHANG Xiao-dong. Phase diagram and thermoldynamic properties of Ag-Cu alloys [J]. Science in China Ser E, 1998, 41(4): 348-356.
[12]PENG Kun. The Systematic Study of The Electronic Structure and Properties of Ti-Al System [D]. Changsha: School of Materials Science and Engineering, Central South University, 2001.
[13]Xie Y Q, Peng K, Liu X B. Influence of xTi/xAl on atomic states, lattice constans and potential-energy planes of ordered Fcc TiAl-type alloys [J]. Physica B, 2004, 344: 5-20.
[14]XIE You-qing, PENG Kun, LIU Xin-bi. Monoatom control designing of disordered hcp TixAl(1-x) alloy [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(4): 205-212.
[15]Lupis C H P. Chemical Thermodynamics of Materials [M]. North-Holland: Amsterdam, 1983. 438-469.
[16]XIE You-qing. A new potential function with many-atom interactions in solid [J]. Science in China Ser A, 1992, 8: 880-890.
[17]Xie Y Q. Electronic structure and properties of pure iron [J]. Acta Metall Mater, 1994, 42(11): 3705-3715.
(Edited by PENG Chao-qun)
Foundation item: Project(50271085) supported by the National Natural Science Foundation of China
Received date: 2004-04-09; Accepted date: 2004-07-23
Correspondence: YU Fang-Xin, PhD candidate; Tel: +86-731-8879287; E-mail: yfx11@263.net