用于增强光电化学水氧化的Sb2O3/Sb2S3/FeOOH复合光阳极的构建
来源期刊:中国有色金属学报(英文版)2020年第6期
论文作者:彭阳 陈佳 蒋良兴 王天意 杨海超 刘芳洋 贾明
文章页码:1625 - 1634
关键词:Sb2O3/Sb2S3;FeOOH助催化剂;光阳极;载流子注入效率
Key words:Sb2O3/Sb2S3; FeOOH co-catalyst; photoanode; carrier injection efficiency
摘 要:通过简单的溶液浸渍法以及化学水浴沉积法和后硫化处理制备新型Sb2O3/Sb2S3/FeOOH光阳极。X射线衍射、拉曼光谱和X射线光电子能谱分析表明成功制备Sb2O3/Sb2S3/FeOOH薄膜。SEM-EDS分析表明,FeCl3溶液浸泡后的Sb2O3/Sb2S3薄膜表面变得粗糙,最优的浸渍时间为8 h。在模拟太阳光和偏压1.23 V(vs RHE)下,FeOOH助催化剂负载的Sb2O3/Sb2S3电极表现出0.45 mA/cm2的光电流密度,为未负载FeOOH光电极的1.41倍。通过紫外-可见光谱、电化学阻抗谱和PEC测试表明,光电性能的提高是由于FeOOH的复合可增强光电极的光捕获能力、降低界面传输阻抗、提高载流子注入效率。
Abstract: A novel Sb2O3/Sb2S3/FeOOH photoanode was fabricated via a simple solution impregnation method along with chemical bath deposition and post-sulfidation. The X-ray diffractometry, Raman measurement, and X-ray photoelectron spectroscopy show that the Sb2O3/Sb2S3/FeOOH thin films are successfully prepared. SEM-EDS analyses reveal that the surface of Sb2O3/Sb2S3 thin films becomes rough after the immersion in the FeCl3 solution. The optimized impregnation time is found to be 8 h. The FeOOH co-catalyst loaded Sb2O3/Sb2S3 electrode exhibits an enhanced photocurrent density of 0.45 mA/cm2 at 1.23 V versus RHE under simulated 1 sun, which is approximately 1.41 times compared to the photocurrent density of the unloaded one. Through the further tests of UV-Vis spectroscopy, the electrochemical impedance spectra, and the PEC measurements, the enhancement can result from the increased light-harvesting ability, the decreased interface transmission impedance, and the remarkably enhanced carrier injection efficiency.
Trans. Nonferrous Met. Soc. China 30(2020) 1625-1634
Yang PENG1, Jia CHEN1,2, Liang-xing JIANG1, Tian-yi WANG1, Hai-chao YANG1, Fang-yang LIU1, Ming JIA1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. BOE Technology Group Co., Ltd., Chengdu 611731, China
Received 8 September 2019; accepted 30 April 2020
Abstract: A novel Sb2O3/Sb2S3/FeOOH photoanode was fabricated via a simple solution impregnation method along with chemical bath deposition and post-sulfidation. The X-ray diffractometry, Raman measurement, and X-ray photoelectron spectroscopy show that the Sb2O3/Sb2S3/FeOOH thin films are successfully prepared. SEM-EDS analyses reveal that the surface of Sb2O3/Sb2S3 thin films becomes rough after the immersion in the FeCl3 solution. The optimized impregnation time is found to be 8 h. The FeOOH co-catalyst loaded Sb2O3/Sb2S3 electrode exhibits an enhanced photocurrent density of 0.45 mA/cm2 at 1.23 V versus RHE under simulated 1 sun, which is approximately 1.41 times compared to the photocurrent density of the unloaded one. Through the further tests of UV-Vis spectroscopy, the electrochemical impedance spectra, and the PEC measurements, the enhancement can result from the increased light-harvesting ability, the decreased interface transmission impedance, and the remarkably enhanced carrier injection efficiency.
Key words: Sb2O3/Sb2S3; FeOOH co-catalyst; photoanode; carrier injection efficiency
1 Introduction
Over the past few decades, the rapid growth of technology has seen the massive consumption of fossil fuels, bringing energy crisis and environmental pollution to the whole world. To exploit clean and sustainable energy resource manufacturing methods is an urgent task for the researchers worldwide [1]. Photoelectrochemical (PEC) water splitting is a fascinating process during which solar energy can be utilized to split water into hydrogen and oxygen [2]. Solar hydrogen aroused widespread interest on account of its representation of clean and efficient energy source. Since FUJISHIMA and HONDA [3] first reported the marvelous hydrogen production ability via TiO2 photoanode in 1972, a great deal of attention had been paid to the artificial photosynthesis especially by the metal oxide semiconductors such as TiO2 [4-6], ZnO [7], WO3 [8], Fe2O3 [9] and Cu2O [10].
Sb2O3, among the various metal oxide semiconductors, has emerged as a highly promising material owing to its excellent chemical stability, non-toxicity, and low cost [11]. Unfortunately, due to its wide band-gap (3-3.6 eV) [12], only a very small part of the solar spectrum can be harvested, which greatly limited its application. Besides, Sb2S3, as another antimony-based semiconductor, is also an original photoelectric material attributed to its suitable energy band-gap (1.7-2.3 eV) and high absorption coefficient (>104 cm-1) [13]. Correspondingly, Sb2S3 has a wide range of applications in the field of solar cells and photo- electrocatalysis [14-17]. DENG et al [14] developed a facile and efficient method to fabricate a full-inorganic epitaxial Sb2S3 solar cell device and obtained an efficiency of 5.4%. DU et al [15] prepared plate-like Mo-doped WO3 films deposited with Sb2S3, which achieved an enhanced photocurrent density of 0.42 mA/cm2 at extremely low input energy (1 mW/cm2), nearly 20 times as high as that of 5%Mo-WO3 film. Besides, DAI et al [17] designed a well-defined hierarchically ordered C-Sb2S3-TNTA ternary photoanode, and with the introduction of Sb2S3 nanocrystals, the solar light harvesting and conversion ability rose to a high level.
Our present work indicates that a Sb2O3/Sb2S3 heterojunction composite structure can be fabricated via chemical bath deposition and post-sulfidation [18]. The composite of Sb2S3 can extend light absorption into the visible region compared to the pure Sb2O3, resulting in an enhanced photocurrent density of 0.35 mA/cm2 at 1.23 V vs RHE under simulated sunlight. However, the obtained photocurrent of the Sb2O3/Sb2S3 thin film is still far less than the theoretically maximum photocurrent of Sb2S3, which is nearly 19 mA/cm2 [19]. Till now, many strategies could be taken to improve the photoelectrochemical performance of a photoelectrode, including doping [20-22], heterojunction construction [23,24], co-catalyst loading [25,26] and plasmonic effect [27]. Given that the heterostructures have already been fabricated, it is feasible that co-catalyst loading could be adopted further to enhance the PEC performance of the Sb2O3/Sb2S3 composite. Recently, widespread interest has been provoked by the iron and nickel oxyhydroxide [28-30] co-catalyst on account of their low overpotential and good contact with the semiconductor interface. In particular, iron oxyhydroxide (FeOOH) has even broader applications in photoanode construction since the iron element is abundant enough in the earth just behind O, Si, and Al, resulting in its low price [31]. For instance, ZHANG et al [32] reported that the deposited FeOOH on nanostructured BiVO4 significantly improved the sluggish kinetics of the oxygen evolution reaction and thus a remarkable photocurrent density of 4.3 mA/cm2 was achieved.
Herein, we demonstrated a facile method to fabricate the Sb2O3/Sb2S3/FeOOH composite photoelectrode. Briefly, the FeOOH nanolayers were later deposited on the surface of the Sb2O3/Sb2S3 composite electrode through the impregnation method after the photoelectrode was prepared by the chemical bath deposition and post-sulfidation. Moreover, the mechanism for the enhancement of the photocurrent was investigated.
2 Experimental
2.1 Preparation of Sb2O3/Sb2S3/FeOOH composite photoelectrode
All chemical reagents in the experiment were of analytical grade and used without further purification. The Sb2O3/Sb2S3 composite thin film was prepared by a chemical bath deposition method followed by post-sulfidation treatment from previous literature [18]. First, a certain amount of triethanolamine was added to form a transparent solution after 0.05 mol/L SbCl3 (99.0%; Aladdin) was dissolved in 60 mL of deionized water. Then, the saturated NaOH (99.0%; Sigma-Aldrich) was added to adjust the pH. After that, the cleaned fluorine-doped tin oxide (FTO) glass slides were vertically immersed into the above precursor solution and maintained at 60 °C for 15 min to form Sb2O3 thin films. After full drying, the as-prepared films were transferred to the tubular furnace, which was in static argon with sulfur vapor mixed atmosphere at the temperature of 350 °C and the pressure of about -0.07 MPa for post-sulfidation treatment.
The FeOOH co-catalysts were prepared by solution impregnation. Thus, the Sb2O3/Sb2S3 photoelectrodes were immersed in the 0.05 mmol/L FeCl3·6H2O (99.0%; Aladdin) solution for varying duration time (6, 8, 10 and 12 h) at room temperature. After the reaction, the obtained thin films were dried in air for several hours.
2.2 Characterization
The crystal structures of the as-prepared samples were identified by an X-ray diffractometer (XRD, Rigaku3014) equipped with Cu Kα radiation (λ=1.54  ). The morphology of the obtained films was observed by a scanning electron microscope (SEM, Zeiss, MERLIN compact at 10 kV accelerating voltage), and the composition was examined by energy-dispersive X-ray spectroscopy (EDX). Raman measurements were carried out on a Jobin Yvon LabRAM HR800 Horiba instrument. The chemical state of the products was probed by X-ray photoelectron spectroscopy (XPS) with a PHI5600 spectrometer. The UV-2450 equipment (SHIMADZU) was applied to evaluating the optical absorption property.
). The morphology of the obtained films was observed by a scanning electron microscope (SEM, Zeiss, MERLIN compact at 10 kV accelerating voltage), and the composition was examined by energy-dispersive X-ray spectroscopy (EDX). Raman measurements were carried out on a Jobin Yvon LabRAM HR800 Horiba instrument. The chemical state of the products was probed by X-ray photoelectron spectroscopy (XPS) with a PHI5600 spectrometer. The UV-2450 equipment (SHIMADZU) was applied to evaluating the optical absorption property.
All PEC measurements were performed with a standard three-electrode system on Princeton Applied Research PARSTAT 4000. In detail, the as-prepared photoanode acted as the working electrode, a high-purity graphite plate as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. The 1.0 mol/L Na2SO4 (pH=5.92) solution was served as the supporting electrolyte. And 1.0 mol/L Na2SO3 (pH=9.82) solution was applied to investigating the carrier injection efficiency. A 300 W xenon lamp with an AM 1.5G filter was adopted to supply the light source. The scan rate of linear sweep voltammetry was 3 mV/s. All potentials relative to SCE were converted to reversible electrode (RHE) values using the Nernst equation:
φRHE=φSCE+0.05916pH+0.2483 (1)
The electrochemical impedance spectra (EIS) were tested at an amplitude of 10 mV with frequency ranging from 0.1 Hz to 100 kHz.
3 Results and discussion
Figure 1(a) shows the XRD patterns of pure Sb2O3, Sb2O3/Sb2S3 and Sb2O3/Sb2S3/FeOOH thin films. For pure Sb2O3, all pronounced diffraction peaks are well assigned to orthorhombic phase β-Sb2O3 (JCPDS No. 11-0689) in addition to the peaks of FTO [11]. After the post-sulfidation treatment, the 2θ angles of 29.2°, 34.4°, 46.7° and 54.4° match those for orthorhombic Sb2S3 [13] (JCPDS No. 42-1393) and only the angle of 44.2° is indexed to Sb2O3, indicating the successful preparation of Sb2O3/Sb2S3 composite material. However, no diffraction peaks of FeOOH are observed after the impregnation of the FeCl3 solution, possibly due to the fact that the as- synthesized FeOOH is amorphous or the content is insufficient. Figure 1(b) displays the Raman spectra of the Sb2O3/Sb2S3 and Sb2O3/Sb2S3/FeOOH photo- electrode. The two sharp peaks at 110 and 147 cm-1 indicate the formation of the Sb2S3 crystalline phase, and the peaks at 281 and 302 cm-1 are consistent with the symmetric vibrations of Sb2S3 pyramidal units [33,34]. Besides, the peaks at 190, 254, 373, 450, 508 and 710 cm-1 are related to the presence of Sb2O3. All signals below 400 cm-1 belong to the external lattice mode regime, while those above 400 cm-1 belong to the internal vibrations [35]. The position of the peaks has hardly changed before and after the impregnation of the FeCl3 solution, which is consistent to the results of XRD patterns. Consequently, other testing methods are required to verify the existence of FeOOH further.
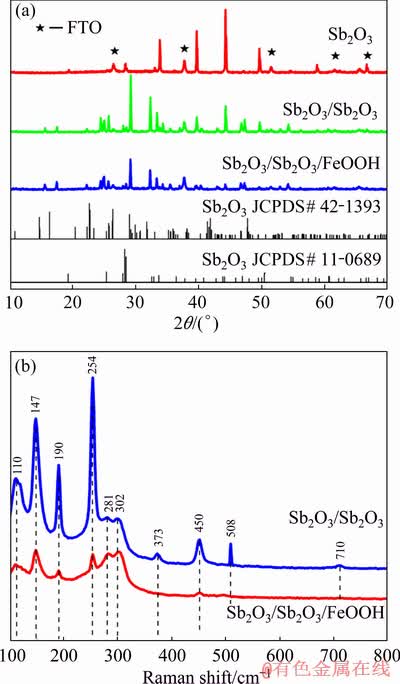
Fig. 1 XRD patterns (a) and Raman spectra (b) of Sb2O3, Sb2O3/Sb2S3 and Sb2O3/Sb2S3/FeOOH thin films
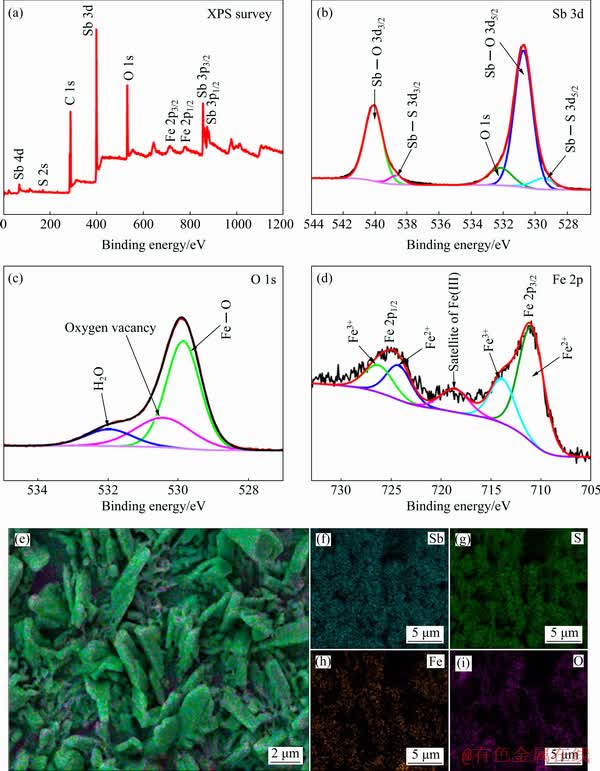
Fig. 2 XPS spectra of Sb2O3/Sb2S3/FeOOH thin films (a-d) and EDX elemental mappings for Sb, S, Fe and O (e-i)
The as-obtained Sb2O3/Sb2S3/FeOOH photo- electrode was later investigated by XPS. The results shown in Fig. 2(a) demonstrate that the elements of Sb, S, C, O and Fe are detected. The peak of C may be derived from CO2 in the atmosphere. The binding energy of C 1s is usually utilized to calibrate the XPS spectrum. Figures 2(b-d) exhibit the high-resolution XPS spectra of Sb 3d, Fe 2p and O 1s. As demonstrated in Fig. 2(b), the peaks at 530.7 eV (Sb 3d5/2) and 540.1 eV (Sb 3d3/2) are indexed to Sb3+ in Sb2O3. Meanwhile, the observed peaks at 529.5 eV (Sb 3d5/2) and 538.7 eV (Sb 3d3/2) correspond to Sb3+ in Sb2S3 [36]. As shown in Fig. 2(c), there are three distinct peaks. Specifically, the peak at 529.8 eV corresponds to the binding between oxygen atoms and metal atoms which refers to the Fe—O bond, the peak at 531.9 eV is associated with the absorption of water molecules, and the peak at 530.4 eV is assigned to the oxygen vacancies. For the Fe 2p core-level spectra (Fig. 2(d)), the binding energies of 711.2 and 714.0 eV are assigned to Fe 2p3/2, and 724.4 and 726.4 eV to Fe 2p1/2, indicating the successful preparation of FeOOH [32]. Also, the relative intensity of Fe2+ is higher than that of Fe3+, which may be attributed to the formation of oxygen vacancies [37]. As a result, the successful preparation of FeOOH can be well illustrated by the XPS analysis.
The energy dispersive X-ray (EDX) analysis was carried out to figure out the elemental distribution of the electrode. As shown in Fig. 2(d), it is clear that the elements of Sb, S, Fe and O evenly distribute on the substrate. Moreover, the weak signal of the Fe element indicates its low content, which is in accordance with the results of XRD and Raman spectra. Hence, it is certain that the FeOOH is undoubtedly loaded onto the surface of the Sb2O3/Sb2S3 photoelectrode by EDX elemental mapping along with the XPS analysis.
The morphology of the as-obtained films is observed by SEM. As presented in Fig. 3(a), the Sb2O3/Sb2S3 thin film is composed of rod-like structures. After the impregnation of the FeCl3 solution, the surface of the sample becomes rough without destroying the original morphology, which may be due to the generated FeOOH covering the surface of the electrode.
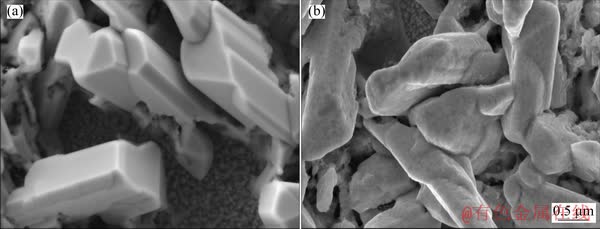
Fig. 3 SEM images of Sb2O3/Sb2S3 (a) and Sb2O3/Sb2S3/FeOOH (b) composite films
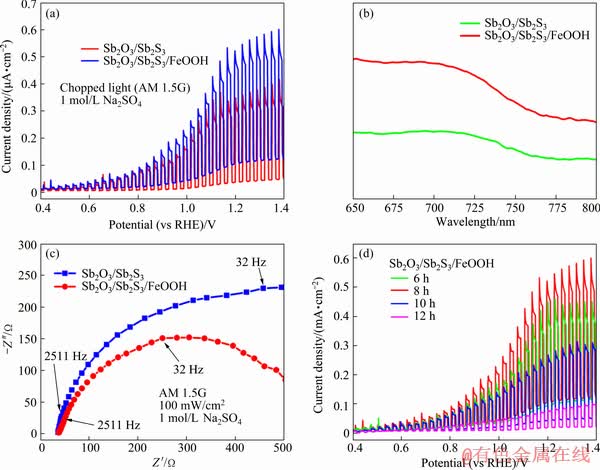
Fig. 4 Photocurrent-potential curves (a), UV-Vis absorption spectra (b), electrochemical impedance spectra (c), and PEC measurements (d) of Sb2O3/Sb2S3 and Sb2O3/Sb2S3/FeOOH photoelectrodes
The photoelectrochemical response of the as-obtained thin films is of great significance for assessing the potential for solar water splitting as a photoanode. Figure 4(a) shows linear voltammetry sweeps on the Sb2O3/Sb2S3 and Sb2O3/Sb2S3/ FeOOH photoanodes under chopped illumination in 1 mol/L Na2SO4 solution. As for the FeOOH-free electrode, the photocurrent is only 0.32 mA/cm2 at 1.23 V versus RHE. Interestingly, an enhanced photocurrent density of 0.45 mA/cm2 over the FeOOH loaded electrode is achieved, which is nearly 1.4 folds of the unloaded one at the same potential. This may be due to the excellent electrocatalytic activity of the FeOOH which can act as a hole transfer layer, thus reducing the recombination rate [38]. Therefore, the kinetics of the oxygen evolution reaction is dramatically accelerated as a result of the increased holes involved in the water oxidation reaction.
To further explore the mechanism of the enhanced photoelectrochemical performance, the optical absorption behavior by using UV-Vis absorbance spectroscopy is shown in Fig. 4(b). Both for Sb2O3/Sb2S3 and Sb2O3/Sb2S3/FeOOH photoanodes, the absorption edge located at around 800 nm remains almost unchanged, while the optical absorption of FeOOH loaded photo- electrodes increases significantly. The improved light absorption for Sb2O3/Sb2S3/FeOOH might be caused by the rough surface (as shown in Fig. 3(b)), which can increase the specific surface area, hence increasing the PEC performance.
Figure 4(c) demonstrates the electrochemical impedance spectra of Sb2O3/Sb2S3 composite photoelectrode before and after the impregnation of the FeCl3 solution. EIS measurements are utilized to evaluate the interface separation and migration properties of the carriers. It can be observed that the FeOOH loaded Sb2O3/Sb2S3 photoelectrode exhibits a smaller radius in the low-frequency region, indicating that the loading of FeOOH can remarkably decrease the resistances in the charge transfer process. In other words, FeOOH co-catalyst accelerates the charge transfer across the interface of the Sb2O3/Sb2S3/FeOOH electrode and the electrolyte, resulting in the suppression of electron-hole recombination. In addition, the results of EIS measurements match the PEC test results well.
The appropriate thickness of photoelectric materials plays a vital role in its photoelectron- chemical performance. On one hand, very thin films may deteriorate the light absorption ability since light can pass through the films easily. On the other hand, excess material may enlarge the carrier diffusion length, thus enhancing the likelihood of carrier recombination. LI et al [38] reported that the hole transfer layer of Fe2O3 deposited on the surface of pure BiVO4 promoted the hole transfer from the bulk of the semiconductor to the electrode surface. Nevertheless, the thickness of the Fe2O3 layer has a great influence on the BiVO4 photoanode owing to the short carrier diffusion length of Fe2O3. So, it might be reasonable to infer that the thickness of the FeOOH layer is one of the decisive factors in the PEC properties of the Sb2O3/Sb2S3/FeOOH composite electrode. Here, the thickness of the FeOOH thin films might be mainly controlled by the immersion time, and the photoelectrochemical performances of Sb2O3/Sb2S3 with varied duration time are shown in Fig. 4(d). It is obvious that the different photoanodes after 6, 8, 10 and 12 h immersion exhibit distinct photocurrents of 0.33, 0.45, 0.24 and 0.07 mA/cm2, respectively, at 1.23 V versus RHE under simulated illumination. The photocurrent density starts to decline after immersing for over 8 h, which agrees with the hypothesis that the thickness does have an impact on photoelectrochemical properties. This is possibly attributed to the thickness of the film exceeding the carrier diffusion length, which leads to an increase in carrier recombination rate and a decrease in PEC properties.
The solar-to-hydrogen (STH) conversion efficiency is a considerable parameter to characterize the photoelectrochemical properties of a photoelectrode. Generally speaking, STH efficiency can be judged by the light absorption efficiency (ηabs), the charge separation efficiency (ηsep) and the charge injection efficiency (ηinj) [39]:
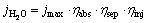 (2)
(2)
where jmax corresponds to the theoretical maximum photocurrent density, while 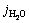 refers to the STH efficiency expressed by current density. Supposing that the PEC measurements are carried out in Na2SO4 solution, the STH efficiency can be expressed by Eq. (3). Na2SO3 as a hole scavenger can immediately consume the holes transferred to the semiconductor/electrolyte interface, which means that the injection efficiency of the photoelectrochemical process is 100%. Then, Eq. (3) can be simplified to Eq. (4) in Na2SO3 solution:
refers to the STH efficiency expressed by current density. Supposing that the PEC measurements are carried out in Na2SO4 solution, the STH efficiency can be expressed by Eq. (3). Na2SO3 as a hole scavenger can immediately consume the holes transferred to the semiconductor/electrolyte interface, which means that the injection efficiency of the photoelectrochemical process is 100%. Then, Eq. (3) can be simplified to Eq. (4) in Na2SO3 solution:
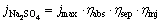 (3)
(3)
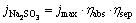 (4)
(4)
The same photoelectrode has the same theoretical maximum photocurrent density. Hence, the injection efficiency (ηinj) in Na2SO4 solution can be obtained via dividing Eq. (3) by Eq. (4):
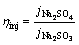 (5)
(5)
The photoelectrochemical measurements of Sb2O3/Sb2S3 and Sb2O3/Sb2S3/FeOOH photo- electrodes were carried out in Na2SO4 and Na2SO3 solution, respectively (as presented in Figs. 5(a) and 5(b)). The photocurrents measured in Na2SO4 and Na2SO3 solution have significant differences, indicating the existence of slow water oxidation kinetics. The net photocurrent density is calculated by simply subtracting the dark current from the light current, and the results are shown in Figs. 5(c) and 5(d). It is worth noting that there is no significant difference between the photocurrent density of Sb2O3/Sb2S3/FeOOH and Sb2O3/Sb2S3 in Na2SO3 aqueous solution, which indicates that the loading of FeOOH co-catalyst can also accelerate the surface oxidation thermodynamics and eliminate the surface recombination of charges, same as the Na2SO3 [40]. The carrier injection efficiency demonstrated in Fig. 5(e) is obtained according to Eq. (4). The ηinj of Sb2O3/Sb2S3/ FeOOH photoelectrode is higher than that of the Sb2O3/Sb2S3 electrode throughout the overall voltage range. The injection efficiency of FeOOH loaded and unloaded electrodes at the bias of 1.10 V versus RHE are 35% and 17%, respectively. This is consistent with the fact that the Sb2S3 has a poor water oxidation kinetics. Consequently, the FeOOH nanolayer serving as co-catalyst can tremendously enhance the carrier injection efficiency, thus accelerating the surface water oxidation kinetics, which is the principal consideration for the enhanced photoelectrochemical performance.
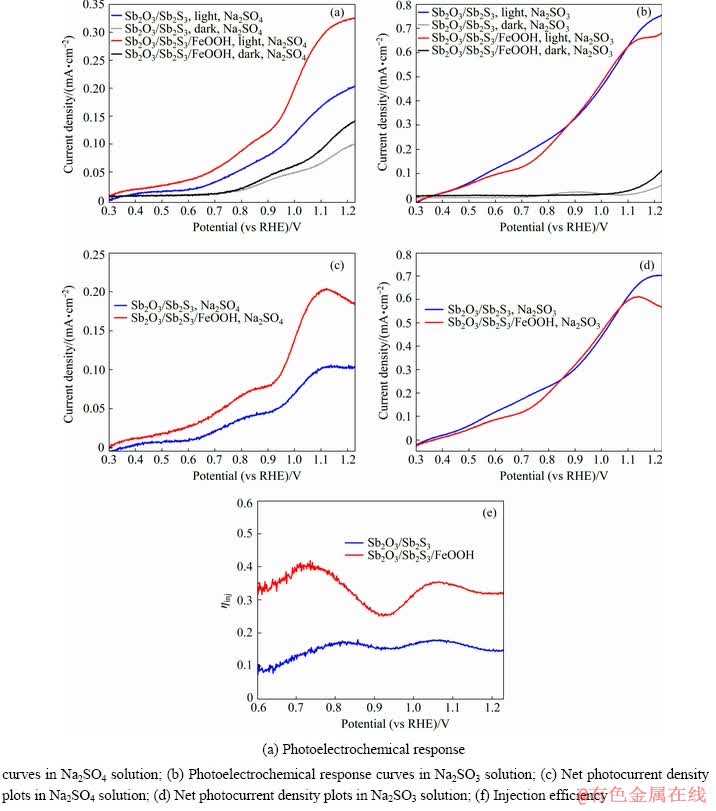
Fig. 5 PEC measurements of Sb2O3/Sb2S3 and Sb2O3/Sb2S3/FeOOH photoelectrodes
4 Conclusions
(1) The Sb2O3/Sb2S3/FeOOH composite photo- electrodes have been successfully fabricated via a simple solution immersion method along with chemical bath deposition and post-sulfidation. And the SEM-EDS analyses reveal that the surface of Sb2O3/Sb2S3 thin films becomes rough after the immersion in the FeCl3 solution.
(2) The Sb2O3/Sb2S3/FeOOH photoelectrode displays an enhanced photocurrent density of 0.45 mA/cm2 at 1.23 V versus RHE in Na2SO4 solution under simulated 1 sun, which is about 1.41 folds that of Sb2O3/Sb2S3 electrode. And the optimized impregnation time is found to be 8 h.
(3) The mechanism of the enhanced photocurrent is investigated by UV-Vis spectroscopy, the electrochemical impedance spectra, and the PEC measurements. And the improved photo-electrochemical performance of Sb2O3/Sb2S3/FeOOH composite photoelectrode is attributed to the increased light absorption capacity, the decreased interface transmission impedance, and the remarkably enhanced carrier injection efficiency.
References
[1] STAMBOULI A B, TRAVERSA E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy [J]. Renewable and Sustainable Energy Reviews, 2002, 6(5): 433-455. https://doi.org/10.1016/ S1364-0321(02)00014-X.
[2] JIANG C, MONIZ S J A, WANG A, ZHANG T, TANG J. Photoelectrochemical devices for solar water splitting– Materials and challenges [J]. Chemical Society Reviews, 2017, 46(15): 4645-4660. https://doi.org/10.1039/ C6CS00306K.
[3] FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 238(5358): 37-38. https://doi.org/10.1038/238037a0.
[4] ZENG Z, LI Y B, CHEN S, CHEN P, XIAO F X. Insight into the charge transport correlation in Aux clusters and graphene quantum dots deposited on TiO2 nanotubes for photoelectrochemical oxygen evolution [J]. Journal of Materials Chemistry A, 2018, 6(24): 11154-11162. https:// doi.org/10.1039/C8TA02802H.
[5] ZENG Z, LI T, LI Y B, DAI X C, HUANG M H, HE Y, XIAO G, XIAO F X. Plasmon-induced photoelectrochemical water oxidation enabled by in situ layer-by-layer construction of cascade charge transfer channel in multilayered photoanode [J]. Journal of Materials Chemistry A, 2018, 6(48): 24686-24692. https://doi.org/10.1039/ C8TA08841A.
[6] ZHANG S, CAO X B, WU J, ZHU L W, GU L. Preparation of hierarchical CuO@TiO2 nanowire film and its application in photoelectrochemical water splitting [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(8): 2094- 2101. https://doi.org/10.1016/S1003-6326(16)64324-8.
[7] KANG Z, SI H, ZHANG S, WU J, SUN Y, LIAO Q, ZHANG Z, ZHANG Y. Interface engineering for modulation of charge carrier behavior in ZnO photoelectrochemical water splitting [J]. Advanced Functional Materials, 2019, 29(15): 1808032. https://doi.org/10.1002/adfm.201808032.
[8] LI Y, LIU Z, ZHANG J, GUO Z, XIN Y, ZHAO L. 1D/0D WO3/CdS heterojunction photoanodes modified with dual co-catalysts for efficient photoelectrochemical water splitting [J]. Journal of Alloys and Compounds, 2019, 790: 493-501. https://doi.org/10.1016/j.jallcom.2019.03.178.
[9] KIM J H, JANG J W, JO Y H, ABDI F F, LEE Y H, VAN DE KROL R, LEE J S. Hetero-type dual photoanodes for unbiased solar water splitting with extended light harvesting [J]. Nature Communications, 2016, 7: 13380. https:// doi.org/10.1038/ncomms13380.
[10] CHEN D, LIU Z, GUO Z, YAN W, XIN Y. Enhancing light harvesting and charge separation of Cu2O photocathodes with spatially separated noble-metal cocatalysts towards highly efficient water splitting [J]. Journal of Materials Chemistry A, 2018, 6(41): 20393-20401. https://doi.org/ 10.1039/C8TA07503D.
[11] DENG Z, CHEN D, TANG F, MENG X, REN J, ZHANG L. Orientated attachment assisted self-assembly of Sb2O3 nanorods and nanowires: End-to-end versus side-by-side [J]. The Journal of Physical Chemistry C, 2007, 111(14): 5325-5330. https://doi.org/10.1021/jp068545o.
[12] CAREY J J, ALLEN J P, SCANLON D O, WATSON G W. The electronic structure of the antimony chalcogenide series: Prospects for optoelectronic applications [J]. Journal of Solid State Chemistry, 2014, 213: 116-125. https://doi.org/10. 1016/j.jssc.2014.02.014.
[13] MEDINA-MONTES M I, MONTIEL-GONZALEZ Z, MATHEWS N R, MATHEW X. The influence of film deposition temperature on the subsequent post-annealing and crystallization of sputtered Sb2S3 thin films [J]. Journal of Physics and Chemistry of Solids, 2017, 111: 182-189. https://doi.org/10.1016/j.jpcs.2017.07.035.
[14] DENG H, ZENG Y, ISHAQ M, YUAN S, ZHANG H, YANG X, HOU M, FAROOQ U, HUANG J, SUN K, WEBSTER R, WU H, CHEN Z, YI F, SONG H, HAO X, TANG J. Quasiepitaxy strategy for efficient full-inorganic Sb2S3 solar cells [J]. Advanced Functional Materials, 2019, 29(31): 1901720. https://doi.org/10.1002/adfm.201901720.
[15] DU H, YANG C, PU W, ZHAO H, GONG J. Highly active Sb2S3-attached Mo–WO3 composite film for enhanced photoelectrocatalytic water splitting at extremely low input light energy [J]. ACS Sustainable Chemistry and Engineering, 2019, 7(10): 9172-9181. https://doi.org/ 10.1021/acssuschemeng. 8b06545.
[16] CAI Q, LIU Z, HAN C, TONG Z, MA C. CuInS2/Sb2S3 heterostructure modified with noble metal co-catalyst for efficient photoelectrochemical water splitting [J]. Journal of Alloys and Compounds, 2019, 795: 319-326. https:// doi.org/10.1016/j.jallcom.2019.04.312.
[17] DAI X C, HUANG M H, LI Y B, LI T, ZHANG B B, HE Y, XIAO G, XIAO F X. Regulating spatial charge transfer over intrinsically ultrathin-carbon-encapsulated photoanodes toward solar water splitting [J]. Journal of Materials Chemistry A, 2019, 7(6): 2741-2753. https://doi.org/10. 1039/ C8TA10379H.
[18] JIANG L, CHEN J, WANG Y, PAN Y, XIAO B, OUYANG N, LIU F. Sb2O3/Sb2S3 heterojunction composite thin film photoanode prepared via chemical bath deposition and post-sulfidation [J]. Journal of the Electrochemical Society, 2018, 165(16): H1052-H1058. https://doi.org/10.1149/ 2.0581816jes.
[19] DEANGELIS A D, KEMP K C, GAILLARD N, KIM K S. Antimony(III) sulfide thin films as a photoanode material in photocatalytic water splitting [J]. ACS Applied Materials and Interfaces, 2016, 8(13): 8445-8451. https://doi.org/10.1021/ acsami.5b12178.
[20] PARMAR K P S, KANG H J, BIST A, DUA P, JANG J S, LEE J S. Photocatalytic and photoelectrochemical water oxidation over metal-doped monoclinic BiVO4 photoanodes [J]. ChemSusChem, 2012, 5(10): 1926-1934. https://doi. org/10.1002/cssc.201200254.
[21] SAGARA N, KAMIMURA S, TSUBOTA T, OHNO T. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light [J]. Applied Catalysis B: Environmental, 2016, 192: 193-198. https://doi.org/10.1016/j.apcatb.2016.03.055.
[22] QIAO L Y, XIE F Y, XIE M H, GONG C H, WANG W L, GAO J C. Characterization and photoelectrochemical performance of Zn-doped TiO2 films by sol–gel method [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(8): 2109-2116. https://doi.org/10.1016/S1003-6326(16) 64325-X.
[23] SU J, GUO L, BAO N, GRIMES C A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting [J]. Nano Letters, 2011, 11(5): 1928-1933. https://doi.org/10.1021/nl2000743.
[24] HOU S, DAI X C, LI Y B, HUANG M H, LI T, WEI Z Q, HE Y, XIAO G, XIAO F X. Charge transfer modulation in layer-by-layer-assembled multilayered photoanodes for solar water oxidation [J]. Journal of Materials Chemistry A, 2019, 7(39): 22487-22499. https://doi.org/10.1039/C9TA08107K.
[25] ZHONG D K, CORNUZ M, SIVULA K, GRATZEL M, GAMELIN D R. Photo-assisted electrodeposition of cobalt–phosphate (Co-Pi) catalyst on hematite photoanodes for solar water oxidation [J]. Energy and Environmental Science, 2011, 4(5): 1759-1764. https://doi.org/10.1039/ C1EE01034D.
[26] NAKADA A, UCHIYAMA T, KAWAKAMI N, SAHARA G, NISHIOKA S, KAMATA R, KUMAGAI H, ISHITANI O, UCHIMOTO Y, MAEDA K. Solar water oxidation by a visible-light-responsive tantalum/nitrogen-codoped rutile titania anode for photoelectrochemical water splitting and carbon dioxide fixation [J]. Chem Photo Chem, 2019, 3(1): 37-45. https://doi.org/10.1002/cptc.201800157.
[27] XIAO F X, LIU B. Plasmon-dictated photo-electrochemical water splitting for solar-to-chemical energy conversion: Current status and future perspectives [J]. Advanced Materials Interfaces, 2018, 5(6): 1701098. https://doi.org/ 10.1002/admi.201701098.
[28] TROTOCHAUD L, YOUNG S L, RANNEY J K, BOETTCHER S W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: The role of intentional and incidental iron incorporation [J]. Journal of the American Chemical Society, 2014, 136(18): 6744-6753. https://doi. org/10.1021/ja502379c.
[29] ABEL A J, PATEL A M, SMOLIN S Y, OPASANONT B, BAXTER J B. Enhanced photoelectrochemical water splitting via SILAR-deposited Ti-doped hematite thin films with an FeOOH overlayer [J]. Journal of Materials Chemistry A, 2016, 4(17): 6495-6504. https://doi.org/10. 1039/ C6TA01862A.
[30] LAN Y, LIU Z, GUO Z, LI X, ZHAO L, ZHAN L, ZHANG M. A ZnO/ZnFe2O4 uniform core–shell heterojunction with a tubular structure modified by NiOOH for efficient photoelectrochemical water splitting [J]. Dalton Transactions, 2018, 47(35): 12181-12187. https://doi.org/10.1039/ C8DT02581A.
[31] YU Q, MENG X, WANG T, LI P, YE J. Hematite films decorated with nanostructured ferric oxyhydroxide as photoanodes for efficient and stable photoelectrochemical water splitting [J]. Advanced Functional Materials, 2015, 25(18): 2686-2692. https://doi.org/10.1002/adfm. 201500383.
[32] ZHANG B, WANG L, ZHANG Y, DING Y, BI Y. Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation [J]. Angewandte Chemie International Edition, 2018, 57(8): 2248-2252. https://doi.org/10.1002/anie.201712499.
[33] PILAPONG C, THONGTEM T, THONGTEM S. Hydrothermal synthesis of double sheaf-like Sb2S3 using copolymer as a crystal splitting agent [J]. Journal of Alloys and Compounds, 2010, 507(2): 38-42. https://doi.org/ 10.1016/j.jallcom.2010.08.003.
[34] BOUGHALMI R, BOUKHACHEM A, KAHLAOUI M, MAGHRAOUI H, AMLOUK M. Physical investigations on Sb2S3 sprayed thin film for optoelectronic applications [J]. Materials Science in Semiconductor Processing, 2014, 26: 593-602. https://doi.org/10.1016/j.mssp.2014.05.059.
[35] MESTL G, RUIZ P, DELMON B, KNOZINGER H. Sb2O3/Sb2O4 in reducing/oxidizing environments: An in situ Raman spectroscopy study [J]. The Journal of Physical Chemistry, 1994, 98(44): 11276-11282. https://doi.org/10. 1021/j100095a008.
[36] LI X, WANG X, NING X, LEI J, SHAO J, WANG W, HUANG Y, HOU B. Sb2S3/Sb2O3 modified TiO2 photoanode for photocathodic protection of 304 stainless steel under visible light [J]. Applied Surface Science, 2018, 462: 155-163. https://doi.org/10.1016/j.apsusc.2018.08.108.
[37] FENG J X, XU H, DONG Y T, YE S H, TONG Y X, LI G R. FeOOH/Co/FeOOH hybrid nanotube arrays as high- performance electrocatalysts for the oxygen evolution reaction [J]. Angewandte Chemie International Edition, 2016, 55(11): 3694-3698. https://doi.org/10.1002/anie.201511447.
[38] LI L, LI J, BAI J, ZENG Q, XIA L, ZHANG Y, CHEN S, XU Q, ZHOU B. Serial hole transfer layers for a BiVO4 photoanode with enhanced photoelectrochemical water splitting [J]. Nanoscale, 2018, 10(38): 18378-18386. https://doi.org/ 10.1039/C8NR06342G.
[39] JEONG H W, JEON T H, JANG J S, CHOI W, PARK H. Strategic modification of BiVO4 for improving photoelectrochemical water oxidation performance [J]. The Journal of Physical Chemistry C, 2013, 117(18): 9104-9112. https://doi.org/10.1021/jp400415m.
[40] ZHONG D K, CHOI S, GAMELIN D R. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W:BiVO4 [J]. Journal of the American Chemical Society, 2011, 133(45): 18370-18377. https://doi.org/10.1021/ja207348x.
彭 阳1,陈 佳1,2,蒋良兴1,王天意1,杨海超1,刘芳洋1,贾 明1
1. 中南大学 冶金与环境学院,长沙 410083;
2. 京东方科技集团有限公司,成都 611731
摘 要:通过简单的溶液浸渍法以及化学水浴沉积法和后硫化处理制备新型Sb2O3/Sb2S3/FeOOH光阳极。X射线衍射、拉曼光谱和X射线光电子能谱分析表明成功制备Sb2O3/Sb2S3/FeOOH薄膜。SEM-EDS分析表明,FeCl3溶液浸泡后的Sb2O3/Sb2S3薄膜表面变得粗糙,最优的浸渍时间为8 h。在模拟太阳光和偏压1.23 V(vs RHE)下,FeOOH助催化剂负载的Sb2O3/Sb2S3电极表现出0.45 mA/cm2的光电流密度,为未负载FeOOH光电极的1.41倍。通过紫外-可见光谱、电化学阻抗谱和PEC测试表明,光电性能的提高是由于FeOOH的复合可增强光电极的光捕获能力、降低界面传输阻抗、提高载流子注入效率。
关键词:Sb2O3/Sb2S3;FeOOH助催化剂;光阳极;载流子注入效率
(Edited by Bing YANG)
Foundation item: Project (51674298) supported by the National Natural Science Foundation of China; Project (2017JJ3384) supported by the Natural Science Foundation of Hunan Province, China; Project (2018M630910) supported by the China Postdoctoral Science Foundation
Corresponding author: Liang-xing JIANG; Tel: +86-731-88830474; E-mail: lxjiang@csu.edu.cn
DOI: 10.1016/S1003-6326(20)65325-0

