DOI: 10.11817/j.issn.1672-7207.2019.01.004
基于石灰石法脱硫浆液的K2S2O8氧化Hg0性能
田立江,刘秉坤,李杰,沈晓玲
(中国矿业大学 环境与测绘学院,江苏 徐州,221116)
摘要:利用改进的Hg0液相氧化反应器,考察脱硫浆液pH、K2S2O8浓度、金属离子浓度、入口烟气Hg0和SO2质量浓度对氧化Hg0性能的影响。研究结果表明:低pH有利于K2S2O8高效氧化Hg0,pH在5.0左右时,Hg0氧化效率能维持在83%以上。K2S2O8浓度在5 mmol/L时,Hg0氧化效率高于90%并呈现很好的运行稳定性。Mn2+,Ag+或Co2+的存在均可提高Hg0氧化性能,但提高K2S2O8浓度至5 mmol/L也能达同等效果。Hg0氧化效率随浆液Fe3+浓度的增加先增加后趋于稳定,当Fe3+浓度增至2.0 mmol/L以上时,Hg0氧化效率再次增加;Mg2+的存在反而使Hg0性能有所降低。Hg0氧化效率随烟气SO2质量浓度的升高先增加后逐渐下降,最高可达85.57%,且SO2的存在增强了系统Hg0氧化的稳定性。
关键词:石灰石-石膏法;脱硫浆液;燃煤烟气;汞氧化
中图分类号:X511 文献标志码:A 文章编号:1672-7207(2019)01-0023-06
Characteristics of Hg0 oxidation by K2S2O8 based on limestone-gypsum desulfurization slurry
TIAN Lijiang, LIU Bingkun, LI Jie, SHEN Xiaoling
(School of Environment Science & Spatial Informatics, China University of Mining and Technology, Xuzhou 221116, China)
Abstract: The effects of slurry pH, K2S2O8 concentration, metallic ion concentration, inlet flue gas Hg0 and SO2 mass concentration on Hg0 oxidation were studied based on the modified liquid reactor. The results show that low value of slurry pH is propitious to oxidize Hg0, and the oxidation efficiency can maintain above 83% when the value of pH keeps around 5.0. The oxidation efficiency of Hg0 can be higher than 90% and keeps stable steadily when K2S2O8 concentration reaches 5 mmol/L. The existence of Mn2+, Ag+ or Co2+ can improve the Hg0 oxidation capability while it can acquire the same outcome through increasing the K2S2O8 concentration to 5 mmol/L. With the increase of Fe3+ concentration, the Hg0 oxidation efficiency rises firstly, then becomes steady, but the efficiency rises again when the Fe3+ concentration is higher than 2.0 mmol/L. The existence of Mg2+ does not enhance the Hg0 oxidation but weakens the oxidation capability. Hg0 oxidation efficiency rises firstly and then descends gradually, and the highest efficiency can reach 85.57% with the increase of flue gas SO2 mass concentration. The existence of SO2 can enhance the stabilization of the system about Hg0 oxidation.
Key words: limestone-gypsum method; desulfurization slurry; coal fired flue gas; mercury oxidation
我国的能源格局长期以来一直以煤炭为主,煤炭的使用量居于世界首位。到2016年,中国原煤产量达34.1亿t,全国发电装机容量为164 575万kW,比上年末增长8.2%,其中火电装机容量105 388万kW,增长5.3%[1]。预计到2020年,全国发电装机容量将达到20亿kW,年均增长5.5%,这将进一步增加煤炭的消耗量和燃煤烟气污染物排放量,同时面临着国家和地方政府对燃煤烟气污染物排放标准的不断严格,特别是新增了对燃煤烟气Hg排放浓度的限值要求[2]。2013年我国“汞污染防治技术政策编制说明”(征求意见稿)数据显示,2007 年汞在大气中的排放量约为643 t,燃煤锅炉和燃煤电厂是最大的大气汞排放源,总计超过50%[3]。因此,我国燃煤锅炉和燃煤电厂的Hg污染物的治理和减排对全国Hg排放总量的控制具有举足轻重的作用。针对我国现有大型燃煤电厂90%以上采用石灰石-石膏湿法脱硫的现状,利用现有脱硫设备实现烟气高效除汞是最为经济合理的途径[4-5]。大量试验结果表明:脱硫浆液虽然能够有效吸收烟气中Hg2+,却很难将Hg0氧化为Hg2+,且被吸收的Hg2+很容易被还原为Hg0再次释放到烟气中[6-9]。因钙基产物对Hg0氧化性能的影响课题组前期试验已有试验和结论[10],所以,本文作者利用前期试验筛选出的促进Hg0氧化性能最佳的氧化剂,着重考察氧化剂浓度、脱硫浆液pH、金属离子和烟气入口Hg0和SO2质量浓度对氧化除汞性能的影响,试验结果有助于利用现有石灰石-石膏湿法脱硫技术实现同时高效脱硫除汞[11]。
1 试验装置与方法
试验系统在原有汞液相氧化反应器基础上加以改进[12],底部增设鼓泡装置以强化气液接触和传质性能;为了满足气液有效接触时间保证充分反应,增加了反应器塔体高度,有效高度达到25 cm,反应器内径为12 cm;同时增设浆液补充和浆液排出系统,保证试验过程中反应器内浆液体积维持在1.5 L,且浆液pH维持稳定,也便于在反应过程中测试浆液组分变化,确保整个试验系统运行更为稳定可靠,试验系统如图1所示。
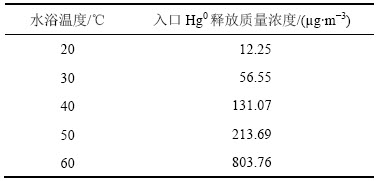
汞源主要由汞渗透管、U形管和恒温水浴加热3部分组成,Hg0质量浓度通过调节恒温水浴温度和载气(N2)流量来实现。Hg0质量浓度采用美国利曼-徕伯斯公司的Hydra II AA 全自动测汞仪进行分析,仪器根据汞质量浓度(μg/m3)响应值自动生成标准曲线,试验要求相关度R2>0.999 9[13]。Hg0质量浓度通过改变水浴温度实现,试验所得水浴温度与Hg0释放质量浓度之间的关系如表1所示。

图1 基于石灰石-石膏湿法脱硫浆液的K2S2O8氧化Hg0试验系统示意图
Fig. 1 Schematic diagram of Hg0 oxidation by K2S2O8 basing on limestone-gypsum desulfurization slurry
表1 Hg0释放质量浓度随水浴温度的变化关系
Table 1 Relationship between mercury release concentration and water bath temperature
2 试验结果与分析
2.1 浆液pH对K2S2O8氧化Hg0性能的影响
在石灰石-石膏湿法脱硫系统运行过程中,浆液pH是影响烟气脱硫效率的关键因素之一,脱硫浆液pH在满足高效脱硫的同时,对Hg0氧化性能影响的研究有助于对现有脱硫系统参数进行调整和优化,同时实现高效脱硫除汞。基于前期研究基础和实际石灰石- 石膏湿法脱硫系统pH运行范围,本阶段试验浆液pH设置在3.0~6.5之间,通过添加质量分数为5%的石灰石浆液调节pH;氧化剂K2S2O8浓度设定为5 mmol/L;为消除周围空气对水浴温度的干扰而影响汞渗透管释放速率,确保试验期间Hg0质量浓度的稳定,同时为了使试验结果更具可比性,Hg源温度设定为50 ℃,Hg源载气流量Q(N2)为1 L/min(下同),鼓泡反应器内浆液体积为1 L(下同),试验结果如图2所示。

图2 脱硫浆液pH对Hg0氧化效率的影响
Fig. 2 Effect of desulfurization slurry pH on Hg0 removal efficiency
由图2可知:当浆液pH为3.14时,系统对Hg0氧化效率达到96.36%,随浆液pH的升高Hg0氧化效率呈下降趋势,当pH达到6.44时,Hg0氧化效率降至78.94%,为试验pH变化范围内氧化除汞效率的最低点。高pH条件下由于OH-的存在,降低了Hg2+转化为Hg(OH)+或Hg(OH)2的可能性,导致大量游离的Hg2+发生还原反应而再次释放到烟气中。低pH条件Hg0氧化效率高的原因是K2S2O8被H+催化,与H2O发生非对称反应,从而导致S=O的断裂,同时浆液还吸收了烟气中部分金属离子如Fe3+和Cu2+等,促使K2S2O8发生金属催化反应,生成一定浓度的O2和H2O2,强化浆液的氧化性能,促进了Hg0的氧化,可能的反应方程式如下[14-15]。
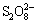 +H++H2O→
+H++H2O→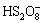 (1)
(1)
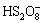 + H2O→
+ H2O→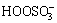 +
+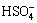 + H+ (2)
+ H+ (2)
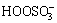 + H2O→
+ H2O→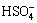 + H2O2 (3)
+ H2O2 (3)
2H2O2→2H2O+O2 (4)
通过脱硫浆液pH对Hg0氧化性能试验结果的分析,结合实际火电机组石灰石-石膏湿法脱硫系统运行的pH运行范围,为实现同时高效脱硫除汞,可将pH控制在5.0左右,Hg0氧化效率可稳定在83%以上。后续试验将脱硫浆液pH均设定在5.0左右。
2.2 K2S2O8浓度对Hg0氧化性能的影响
为考察不同K2S2O8浓度对系统Hg0氧化性能的影响,以及系统运行的稳定性,结合前期试验结论,将试验汞源温度定为50 ℃,反应温度为室温(10 ℃,冬季)。因K2S2O8在常温下较为稳定、初始反应缓慢,故在反应进行10 min后开始采样,不同浓度K2S2O8下的Hg0氧化效率如图3所示。
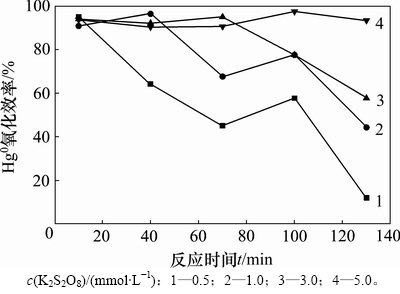
图3 不同浓度K2S2O8条件下反应时间对Hg0氧化效率的影响
Fig. 3 Effect of reaction time on mercury oxidation efficiency with different K2S2O8 concentrations
由图3可知:当K2S2O8浓度为0.5 mmol/L时,Hg0氧化效率随反应时间的延长逐渐降低,在反应至70 min处开始出现小幅上升,上升的原因可能是反应产物对汞发生快速吸附作用[16];反应100 min后Hg0氧化效率快速降低,反应至130 min时,Hg0氧化效率仅为12%。当初始K2S2O8浓度分别为1 mmol/L和3 mmol/L时,Hg0氧化效率分别由反应10 min时的90.82%和94.04%下降到反应130 min时的44.28%和57.70%,下降幅度分别为51.24%和38.64%;当初始K2S2O8浓度到达5 mmol/L时,Hg0氧化效率由反应10 min时的93.68%减小到反应130 min时的93.39%,仅降低了0.29%,反应随时间变化较为稳定。同时在反应时间t为100 min处,出现了试验阶段Hg0氧化效率最高点97.48%,在试验时间内,Hg0氧化效率均在90%以上,并呈现很好的运行稳定性。经综合考虑,后续试验除金属离子强化K2S2O8氧化Hg0试验以外,K2S2O8浓度均设定为5 mmol/L。
2.3 金属离子强化K2S2O8氧化Hg0性能的影响
脱硫浆液中金属离子(Fe3+,Mn2+,Ag+,Co2+和Mg2+)的存在能够催化K2S2O8产生比·OH氧化性更强的·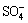 。金属离子催化K2S2O8生成的·
。金属离子催化K2S2O8生成的·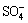 可快速将Hg0氧化为Hg2+进入脱硫浆液。结合实际石灰石-石膏湿法脱硫浆液中金属离子的浓度范围和诸多文献研究结论,以及提高各组试验效果的可对比性,基于前期研究结论,本研究设定Mn2+,Ag+和Co2+ 的浓度均为0.05 mol/L,Mg2+和Fe3+的浓度范围在0.5~5.0 mmol/L之间。试验先考察Mn2+,Ag+和Co2+ 3种金属离子的存在对K2S2O8氧化Hg0性能的影响。
可快速将Hg0氧化为Hg2+进入脱硫浆液。结合实际石灰石-石膏湿法脱硫浆液中金属离子的浓度范围和诸多文献研究结论,以及提高各组试验效果的可对比性,基于前期研究结论,本研究设定Mn2+,Ag+和Co2+ 的浓度均为0.05 mol/L,Mg2+和Fe3+的浓度范围在0.5~5.0 mmol/L之间。试验先考察Mn2+,Ag+和Co2+ 3种金属离子的存在对K2S2O8氧化Hg0性能的影响。
2.3.1 Mn2+,Ag+和Co2+对K2S2O8氧化Hg0性能的影响
本阶段试验汞源温度仍为50 ℃,为使反应结果更具可比性,3种金属离子分别与3 mmol/L K2S2O8进行组合,Hg0氧化效果则分别与3 mmol/L和5 mmol/L K2S2O8氧化效果进行比较,其他试验参数设置同上,试验结果如图4所示。

图4 Mn2+,Ag+和Co2+对K2S2O8氧化Hg0性能的影响
Fig. 4 Effect of Mn2+, Ag+ and Co2+ on mercury oxidation efficiency by K2S2O8
由图4可知:脱硫浆液中Mn2+,Ag+和Co2+的存在不仅可以提高K2S2O8氧化Hg0性能,还能增加系统稳定性。初始K2S2O8浓度为3 mmol/L、反应进行至70 min时,Hg0氧化效率明显下降;当浆液中分别加入一定浓度的金属离子催化剂(c(Ag+)=0.05 mol/L、c(Co2+)=0.05 mol/L或c(Mn2+)=0.05 mol/L)时,在整个试验时间内,系统都维持高效Hg0氧化状态,但相互间差异不大。这是因为金属离子的加入降低了K2S2O8氧化Hg0反应的能垒,使在冷态环境下较为稳定的K2S2O8受到激发,短时间内增加了浆液中处于活化状态的K2S2O8浓度,同时·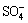 等自由基的生成加快了反应进程。以Ag+为例,可能的反应机理如下[17]。
等自由基的生成加快了反应进程。以Ag+为例,可能的反应机理如下[17]。
Ag++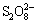 →Ag2++
→Ag2++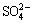 + ·
+ ·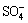 (5)
(5)
Ag2++OH-→Ag++ ·OH (6)
虽然加入金属离子能够提升系统氧化Hg0性能,但适量提高浆液中K2S2O8浓度(5 mmol/L)也能达到同等效果,系统氧化Hg0效率维持在89%以上,还可抑制Hg2+的还原[18]。且浆液中加入的金属离子会随反应进行最终进入脱硫副产物石膏中,延长石膏晶体诱导时间,抑制了石膏结晶,影响石膏晶体的形态,降低了石膏脱水性能[19]。
2.3.2 Mg2+和Fe3+对K2S2O8氧化Hg0性能的影响
石灰石-石膏湿法脱硫系统在实际运行过程中,往往会在脱硫浆液中加入一定浓度的Mg2+以促进石灰石的溶解,提高系统脱硫能力。同时脱硫浆液中含有少量的Fe3+,Fe2+甚至Fe,在采用强制氧化技术或者加入汞氧化剂时,浆液中Fe2+或Fe会氧化为Fe3+。试验分别考察浆液中Mg2+和Fe3+对K2S2O8氧化Hg0性能的影响,试验条件与2.3.1一致,试验结果如图5所示。
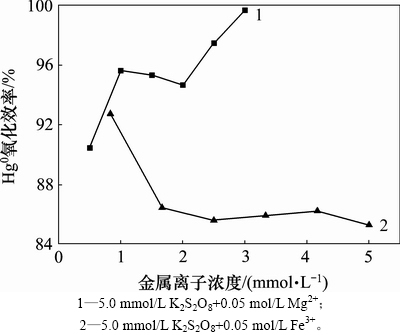
图5 Mg2+和Fe3+对K2S2O8氧化Hg0性能的影响
Fig. 5 Effect of Mg2+ and Fe3+ on mercury oxidation efficiency by K2S2O8
由图5可知:与图2所示仅含K2S2O8(5 mmol/L)浆液的Hg0氧化效率相比,随浆液中Mg2+的加入Hg0氧化效率有所降低。浆液中Mg2+浓度由0.83 mmol/L(即100 mg/L)增加到1.67 mmol/L(即200 mg/L)时,Hg0氧化效率由92.75%降至86.46%,降幅达到6.29%。其原因是Mg2+与亚硫酸根形成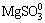 中性离子对,能有效降低液相游离态的活性
中性离子对,能有效降低液相游离态的活性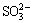 浓度。低浓度的活性
浓度。低浓度的活性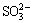 与Hg结合生成HgSO3,HgSO3的稳定性远低于
与Hg结合生成HgSO3,HgSO3的稳定性远低于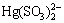 的稳定性,即HgSO3的Hg0释放速度明显比
的稳定性,即HgSO3的Hg0释放速度明显比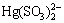 的高。因此,浆液中Hg0释放速度随着浆液中
的高。因此,浆液中Hg0释放速度随着浆液中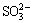 浓度的减低而升高[6]。
浓度的减低而升高[6]。
在K2S2O8+Fe3+系统中,随浆液中Fe3+浓度的增加,系统氧化Hg0性能得到提升,并维持高于94%的Hg0氧化效率。浆液中Fe3+浓度由0.5 mmol/L(即100 mg/L)增至1.0 mmol/L(即200 mg/L),Hg0氧化效率由90.47%提升至95.65%,继续增加浆液中Fe3+浓度效率趋于稳定,这主要是因为Fe3+的加入,对Hg2+还原产生抑制作用,当浆液中Fe3+浓度增加到1.0 mmol/L时,浆液中Hg2+还原性能已经得到有效抑制,继续增加Fe3+浓度,对Hg2+还原性能抑制并不明显,系统氧化Hg0效率较为稳定。当浆液中Fe3+浓度增至2.0 mmol/L(400 mg/L)以上时,Hg0氧化效率再次呈现上升趋势,其原因主要是浆液中高浓度的Fe3+和亚硫酸根不能大量共存,会发生离子间的氧化还原反应, Fe3+将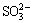 氧化成
氧化成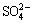 ,反应方程式为
,反应方程式为
2Fe3++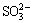 +H2O→2Fe2++
+H2O→2Fe2++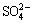 +2H+ (7)
+2H+ (7)
从反应方程式(7)可以看出:高浓度的Fe3+与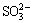 反应后浆液中
反应后浆液中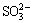 浓度降低,系统的pH也不断降低,导致浆液
浓度降低,系统的pH也不断降低,导致浆液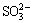 与
与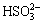 浓度比降低,抑制了Hg0的释放速率,从而提高了系统的Hg0氧化性能[7]。
浓度比降低,抑制了Hg0的释放速率,从而提高了系统的Hg0氧化性能[7]。
2.4 Hg0和SO2质量浓度对K2S2O8氧化Hg0性能的影响
实际烟气组分较为复杂,主要包括未被捕集的飞灰、NOx、O2、重金属和SO2等。在实际工程运行期间,Hg0和SO2的质量浓度变化范围较大,Hg0和SO2的存在相互之间都会产生影响[20-22]。本阶段考察不同质量浓度Hg0和SO2对氧化性能的影响。当试验汞源温度分别为30,50和60 ℃(相应的入口Hg0释放质量浓度见表1),入口SO2质量浓度在100~1 600 mg/m3之间,液气比为17 L/m3,空塔风速v为0.275 m/s时,试验结果如图6所示。
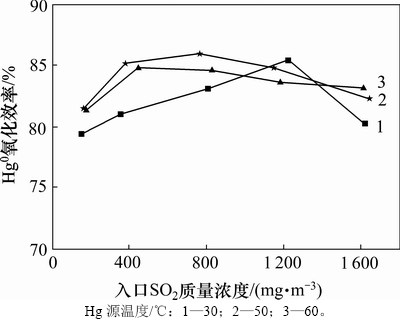
图6 3种Hg0质量浓度条件下入口SO2质量浓度对Hg0氧化效率的影响
Fig. 6 Effect of SO2 mass concentration on mercury oxidation efficiency with three Hg0 mass concentrations
由图6可知:当汞源温度为30 ℃时,随入口SO2质量浓度的增加,Hg0氧化效率先递增后逐渐下降,Hg0氧化效率最高可达85.57%,与试验范围内最低效率相比仅降低6.11%。汞源温度为50 ℃和60 ℃时,SO2质量浓度变化对Hg0氧化效率的影响类似。SO2质量浓度较低(小于1 428 mg/m3)时,随SO2质量浓度的增加,系统氧化Hg0效率得到小幅提升,其原因是SO2质量浓度的不断提高,增加了脱硫浆液中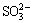 浓度,促进了HgSO3向
浓度,促进了HgSO3向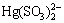 的转化,与HgSO3相比,
的转化,与HgSO3相比,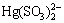 更稳定,分解释放Hg0的速度要慢得多[7]。继续增加SO2质量浓度,系统氧化Hg0效率出现缓慢下降,表明脱硫浆液中过量的SO32-浓度对Hg0氧化已无明显促进作用。从图6还可知:3种汞源温度条件下Hg0氧化效率变化并不明显,其可能的原因是5 mmol/L的K2S2O8氧化Hg0能力最佳,且该浓度条件下的浆液氧化Hg0容量远远高于试验期间进入浆液中被氧化的Hg0总量。由此得出结论,试验条件所设定3种不同质量浓度Hg0烟气对K2S2O8氧化Hg0性能无明显影响,SO2的存在提高了脱硫浆液中K2S2O8氧化Hg0的稳定性。
更稳定,分解释放Hg0的速度要慢得多[7]。继续增加SO2质量浓度,系统氧化Hg0效率出现缓慢下降,表明脱硫浆液中过量的SO32-浓度对Hg0氧化已无明显促进作用。从图6还可知:3种汞源温度条件下Hg0氧化效率变化并不明显,其可能的原因是5 mmol/L的K2S2O8氧化Hg0能力最佳,且该浓度条件下的浆液氧化Hg0容量远远高于试验期间进入浆液中被氧化的Hg0总量。由此得出结论,试验条件所设定3种不同质量浓度Hg0烟气对K2S2O8氧化Hg0性能无明显影响,SO2的存在提高了脱硫浆液中K2S2O8氧化Hg0的稳定性。
3 结论
1) 低pH脱硫浆液有利于K2S2O8高效氧化Hg0,Hg0氧化效率最高可达96.36%,并随浆液pH的升高呈下降趋势。浆液pH在5.0左右时,Hg0氧化效率虽有所降低,但仍能维持在83%以上。
2) 当K2S2O8浓度为0.5,1.0和3.0 mmol/L时,Hg0氧化效率变化幅度较大,最高可达50%以上。当K2S2O8浓度为5 mmol/L时,Hg0氧化性能较稳定,氧化效率均维持在90%以上。
3) 浆液中Mn2+,Ag+或Co2+的存在可提高系统Hg0氧化性能和系统稳定性,但三者间差异不大,适量提高浆液中K2S2O8浓度也能达到同等效果;Mg2+的加入,系统Hg0氧化性能反而有所降低。Hg0氧化效率随Fe3+浓度的增加呈先增加后趋于稳定,当Fe3+浓度增至2.0 mmol/L以上时,Hg0氧化效率再次增加。
4) 试验条件下不同质量浓度Hg0烟气对K2S2O8氧化Hg0性能无明显影响;随入口烟气SO2质量浓度的增加,Hg0氧化效率先增加后逐渐下降,Hg0氧化效率最高可达85.57%,SO2的存在可提高脱硫浆液中K2S2O8氧化Hg0的稳定性。
5) 利用K2S2O8氧化Hg0具有较高的氧化效果,且运行较为稳定。在实际应用中,若浆液成分较为简单,浆液中还原性成分偏少,对浆液中其他成分的存在形式变化无特殊要求,则该方法的应用价值较高。若浆液中存在较高浓度的还原性成分,则会额外消耗氧化剂用量,且会与Hg0存在竞争影响。若应用环境对浆液中还原性成分的存在形式或浓度有特殊要求 时,则在使用过程中需考虑K2S2O8氧化所带来的影响。
参考文献
[2017-02-28]. http://www.stats.gov.cn.
[2] 中国环境保护部. 国家重金属污染综合防治“十二五”规划[EB/OL]. [2011-02-19]. http://www.zhb.gov.cn.
China Environmental Protection Department. Comprehensive prevention and control of heavy metal pollution in the state “Twelfth Five Year Plan”[EB/OL]. [2011-02-19]. http://www. zhb.gov.cn.
[3] 中国环境保护部.《汞污染防治技术政策编制说明》征求意见稿[EB/OL]. [2013-12-08]. http://www.zhb.gov.cn/gkml/hbb/bgth/ 201301/ t20130123_245431.htm.
China Environmental Protection Department. Draft of technical policy for prevention and control of mercury pollution[EB/OL]. [2013-12-08]. http://www.zhb.gov.cn/gkml/hbb/bgth/201301/ t20130123_ 245431.htm.
[4] 梁大镁. 湿法脱硫系统协同脱除汞的实验研究[D]. 武汉: 华中科技大学煤燃烧国家重点实验室, 2011: 27-35.
LIANG Damei. Experimental study of mercury removal with wet flue gas desulfurization system[D]. Wuhan: Huazhong University of Science and Technology. State Key Laboratory of Coal Combustion, 2011: 27-35.
[5] 武成利. 燃煤烟气中汞再析出及抑制研究[D]. 淮南: 安徽理工大学化学工程学院, 2010: 75-112.
WU Chengli. Study on the re-emission and suppression of mercury in the flue gas[D]. Huainan: Anhui University of Science and Technology. School of Chemical Engineering, 2010: 75-112.
[6] 王岳军. 气相零价汞催化氧化及二价汞液相吸收、还原过程研究[D]. 杭州: 浙江大学环境与资源学院, 2011: 32-62.
WANG Yuejun. Catalytic oxidation of gas-phase elemental mercury and bivalent mercury aqueous absorption-reduction behavior study[D]. Hangzhou: Zhejiang University. College of Environmental and Resource, 2011: 32-62.
[7] 陈传敏, 张建华, 俞立. 湿法烟气脱硫浆液中汞再释放特性研究[J]. 中国电机工程学报, 2011, 31(5): 48-51.
CHEN Chuanmin, ZHANG Jianhua, YU Li. Study on the characteristics of mercury reemission from wet flue gas desulfurization solution[J]. Proceedings of the CSEE, 2011, 31(5): 48-51.
[8] SUN Mingyang, LOU Zimo, CHENG Guanghuan. Process migration and transformation of mercury in simulated wet flue gas desulfurization slurry system[J]. Fuel, 2015, 140: 136-142.
[9] WANG Yunjun, DUAN Yufeng, YANG Liguo. Experimental study on mercury transformation and removal in coal-fired boiler flue gases[J]. Fuel Processing Technology, 2009, 90: 643-651.
[10] 田立江, 张甜甜, 王艳芳, 等. 脱硫浆液钙基组分对K2S2O8氧化Hg0的影响[J]. 中国矿业大学学报, 2016, 45(4): 779-784.
TIAN Lijiang, ZHANG Tiantian, WANG Yanfang, et al. Research on Hg0 oxidation by K2S2O8 based on the Ca-based components of desulfurization slurry[J]. Journal of China University of Mining and Technology, 2016, 45(4): 779-784.
[11] 康新园. 燃煤烟气脱硫脱硝一体化技术研究进展[J]. 洁净煤技术, 2014, 20(6): 115-118.
KANG Xinyuan. Research progress of coal-fired flue gas simultaneous desulfurization and denitrification[J]. Clean Coal Technology, 2014, 20(6): 115-118.
[12] 刘嘉宇, 刘亚明,郝雅洁, 等. 湿法脱硫塔内脱硫浆液运动特性[J]. 中南大学学报(自然科学版), 2016, 47(1): 330-337.
LIU Jiayu, LIU Yaming, HAO Yajie, et al. Motion characteristics of gypsum slurry in wet-type desulphurization tower[J]. Journal of Central South University(Science and Technology), 2016, 47(1): 330-337.
[13] YE Zhuang, PAVLISH J H, LENTZ N B. Mercury measurement and control in a CO2-enriched flue gas[J]. International Journal of Greenhouse Gas Control, 2011, 5(S1): S136-S142.
[14] FARR S, HEIDEL B, HILBER M, et al. Influence of flue gas components on mercury removal and retention in dual loop flue gas desulfurization[J]. Energy and Fuels, 2015, 29: 4418-4427.
[15] BAWN C E H, MARGERISON D. Molecular dissociation processes in solution. Part 4: the rate of decomposition of persulphate ion and its catalysis by metal ions[J]. Transactions of the Faraday Society, 1955, 51: 925-934.
[16] 睢辉, 张梦泽, 董勇, 等. 燃煤烟气中单质汞吸附与氧化机理研究进展[J]. 化工进展, 2014, 33(6): 1582-1588.
SUI Hui, ZHANG Mengze, DONG Yong, et al. Research progress of adsorption and oxidation mechanism of elemental mercury from coal-fired flue gas[J]. Chemical Industry and Engineering Progress, 2014, 33(6): 1582-1588.
[17] WU Hui, ZHANG Bi, QIU Yong, et al. Experimental study on mercury migration across wet flue gas desulfurization slurry under oxy-coal combustion atmosphere[J]. Fuel, 2016, 181: 1184-1190.
[18] HEIDEL B, HILBER M, SCHEFFKNECHT G. Impact of additives for enhanced sulfur dioxide removal on re-emissions of mercury in wet flue gas desulfurization[J]. Applied Energy, 2014, 114: 485-491.
[19] SUN Mingyang, HOU Jiaai, CHENG Guanghuan. The relationship between speciation and release ability of mercury in flue gas desulfurization (FGD) gypsum[J]. Fuel, 2014, 125: 66-72.
[20] CHENG Chinmin, CAO Yan, KAI Zhang. Co-effects of sulfur dioxide load and oxidation air on mercury re-emission in forced-oxidation limestone flue gas desulfurization wet scrubber[J]. Fuel, 2013, 106: 505-511.
[21] TAO Ye, ZHUO Yuqun, ZHANG Liang. Impact of flue gas species and temperature on mercury oxidation[J]. Tsinghua Science and Technology, 2010, 15(4): 418-425.
[22] MA Yongpeng, QU Zan, XU Haomiao. Investigation on mercury removal method from flue gas in the presence of sulfur dioxide[J]. Journal of Hazardous Materials, 2014, 279: 289-295.
(编辑 杨幼平)
收稿日期:2018-01-31;修回日期:2018-05-15
基金项目(Foundation item):国家自然科学基金资助项目(51208502) (Project(51208502) supported by the National Natural Science Foundation of China)
通信作者:田立江,博士,副教授,从事烟气除尘脱硫脱硝除汞技术研究;E-mail: tljiang77@163.com