J. Cent. South Univ. (2012) 19: 2447-2450
DOI: 10.1007/s11771-012-1295-8
Synthesis of ethoxycarbonyl isothiocyanate by orthogonal design
LIANG wen-jie(梁文杰)1,2, ZHONG Hong(钟宏)1,2, HE Mou-hai(何谋海)1,2
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Resources Chemistry of Nonferrous Metals (Central South University), Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: Using Schiff base as a phase transfer catalyst, ethoxycarbonyl isothiocyanate was synthesized by reacting ethyl chloroformate with sodium thiocyanate. In order to get the best synthetic technology, an orthogonal test (L9(34)) was applied. The results show that reaction temperature, reaction time, content of catalyst and molar ratio of sodium thiocyanate to ethyl chloroformate are the main factors influencing the yield. The four factors chosen for the present investigation are based on the results of a single-factor test. The optimum synthetic technology is determined as follows: reaction temperature 35 ℃, reaction time 3 h, the content of catalyst (molar fraction based on ethyl chloroformate) 1.5% and molar ratio of sodium thiocyanate to ethyl chloroformate 1.1. Under the optimized synthetic technology, the experimental yield reaches 96.8%.
Key words: ethoxycarbonyl isothiocyanate; Schiff base; phase transfer catalyst; orthogonal test
1 Introduction
Isothiocyanate and its derivatives are important organic synthesis intermediates, which can participate in a variety of organic reactions for the synthesis of various types of sulfurous, nitrogenous and oxygenous organic compounds, and they are also widely used in pesticides, pharmaceuticals, dyes and other products prepared by organic synthesis [1-5]. Ethoxycarbonyl isothiocyanate (ECIT), the main representative of alkoxycarbonyl isothiocyanates, has been proved to be a very useful synthetic reagent which is particularly suitable for the construction of heterocyclic compounds [6]. At present, ECIT is synthesized by reacting ethyl chloroformate with sodium thiocyanate, using phase transfer catalysis synthesis [7]. The phase transfer catalyst is the key technology, which plays an extremely important role. American Cyanamid Company and Bayer Corporation have applied patents for the production of ECIT and its derivatives, respectively [8-9]. American Cyanamid Company uses a catalyst comprising a six-membered mononuclear or ten-membered, fused, polynuclear, aromatic, heterocyclic compound having one or two nitrogen atoms, and Bayer company uses N,N- dialkylarylamine as catalyst. However, most of these aromatic amine catalysts are toxic.
Schiff bases are condensation products of primary amines with carbonyl compounds and they are also known as anils, imines or azomethines. Because of the relative easiness of preparation, synthetic flexibility, and special property of C=N group, Schiff bases have a wide variety of applications in many fields [10-11]. Recent studies showed that Schiff base and its complexes have emerged as highly efficient catalysts in various fields of synthesis and other useful reactions [12-14]. According to Schiff base structure and preliminary experiment, Schiff base is expected to become a highly-efficient catalyst for phase transfer catalysis synthesis of ECIT.
In this work, the synthesis of ECIT using Schiff base as a phase transfer catalyst was studied. The whole reaction process has the advantages of simple operation, mild condition, and at the same time, high yield. The process basically belongs to the high efficient green synthesis method. The reaction is
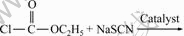
 (1)
(1)
2 Experimental
2.1 Materials
Infrared spectra were measured on a 550 FT-IR spectrometer with samples prepared as KBr pellets. Elements were identified on a Vario EL elemental analyzer. Nuclear magnetic resonance spectra were obtained on a FT-80A instrument in DMSO-d6 with Me4Si as internal standard. Ethyl chloroformate, sodium thiocyanate, cyclohexanone, 3-(dimethylamino)-1- propylamine and benzene were purchased, and all solvents and chemicals were of analytical grade. Doubly distilled water was used throughout the experiment.
2.2 Preparation of catalyst
The Schiff base catalyst was prepared by the reaction between 0.11 mol cyclohexanone and 0.1 mol 3-(dimethylamino)-1-propylamine in benzene at 120 ℃ for 2 h. During the reaction, the generated water was brought out of the reaction mixture by benzene. After completion of the reaction, the reaction solvent benzene was distilled under vacuum, yielding the Schiff base catalyst (94.4% yield). Using KBr pellet method, the Schiff base catalyst was characterized by IR spectral analysis. IR spectra of Schiff base showed a strong band around 1 661 cm-1 for the —CH=N— group stretching. The characteristic absorption peaks of C=O and —NH2 were not observed, indicating that the condensation of the carbonyl with the amino had generated imine, i.e. Schiff base.
2.3 Procedure for synthesis of ethoxycarbonyl isothiocyanate
The 15 mL cyclohexanone was added to a 100 mL round-bottomed flask, and then a certain amount of sodium thiocyanate and Schiff base catalyst were added. After setting reaction temperature, 0.05 mol ethyl chloroformate was added dropwise with stirring over 40 min. Following this addition step, the reaction mixture was stirred at this temperature for a certain time. After completion of the reaction, 20 mL water was added, and the mixture was separated into an aqueous phase and an organic phase. The desired ECIT product was contained within the organic phase. After drying over MgSO4, the organic layer was distilled under vacuum, forming red brown oily liquid of ECIT. The content of ECIT was determined according to Ref. [15].
2.4 Optimization of synthetic conditions of ethoxycarbonyl isothiocyanate
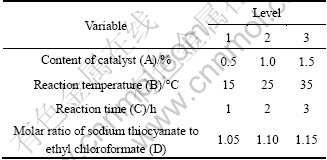
An orthogonal L9(34) test was used to investigate the optimal synthetic condition of ECIT. As seen from Table 1, the synthetic experiment was carried out with four factors and three levels, namely the content of catalyst (A), reaction temperature (B), reaction time (C) and the proportion of material (D). The range of each factor level was based on the results of preliminary experiments. The yield (%) of ECIT was the dependent variable. The ECIT obtained from the above nine tests was operated following the method in Section 2.2.
Table 1 Factors and levels for orthogonal test
3 Results and discussion
3.1 Effect of amount of catalyst
The yield (%) of ECIT affected by different contents of catalyst is shown in Fig. 1, when other three factors (reaction temperature, reaction time and molar ratio of sodium thiocyanate to ethyl chloroformate) are fixed at 35 ℃, 3 h and 1.1, respectively. The content of catalyst presented in the reaction mixture is from 0.25% to 2.0% by molar fraction based on the ethyl chloroformate. It can be seen that the yield of ECIT increases with the content of catalyst increasing. However, when the content of catalyst increases to 1.5%, the yield no longer increases.
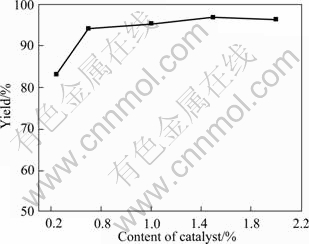
Fig. 1 Relationship between content of catalyst and yield
3.2 Effect of molar ratio of sodium thiocyanate to ethyl chloroformate
In this work, the effect of molar ratio of sodium thiocyanate to ethyl chloroformate on yield of ECIT was investigated, and the results are shown in Fig. 2. Firstly, other synthetic conditions, e.g. reaction temperature, reaction time and content of catalyst (molar fraction based on ethyl chloroformate) are fixed at 35 ℃, 3 h and 1.5%, respectively, and only molar ratio of sodium thiocyanate to ethyl chloroformate changes. As shown in Fig. 2, the yield of ECIT continues to increase with increasing the molar ratio of sodium thiocyanate to ethyl chloroformate and reaches the peak value when molar ratio of sodium thiocyanate to ethyl chloroformate is 1.1. With further increasing the molar ratio of sodium thiocyanate to ethyl chloroformate, the yield no longer increases.

Fig. 2 Relationship between proportion of material and yield
3.3 Effect of reaction temperature and reaction time
The yields (%) of ECIT affected by different reaction temperatures and different reaction time are shown in Fig. 3, when other two factors (the content of catalyst and molar ratio of sodium thiocyanate to ethyl chloroformate) are fixed at 1.5% and 1.1. As shown in Fig. 3, elevated temperature is beneficial to not only improving the catalytic synthesis reaction yield, but also accelerating the reaction rate. Figure 3 also shows that the yield increases with increasing the reaction time under the condition of the same reaction temperature. At low reaction temperature (15 ℃), the reaction rate and
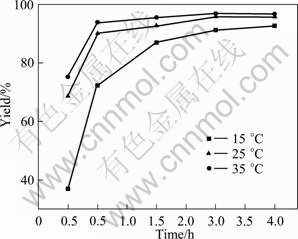
Fig. 3 Relationship between reaction time and yield at different reaction temperatures
the reaction yield are relatively low. When the reaction temperature rises to 25 ℃, the reaction rate significantly increases, and the reaction yield is also significantly improved. With further increasing the reaction temperature to 35 ℃, the reaction yield reaches 93.8% after 1 h, and a maximum value of 96.8% is obtained after 3 h, which is higher than that at 15 ℃ and 25 ℃. With further increasing the reaction time at 35 ℃, the reaction yield has little change.
Thus, the content of catalyst (molar fraction based on ethyl chloroformate) of 0.5%-1.5%, reaction temperature of 15-35 ℃, reaction time of 1-3 h and molar ratio of sodium thiocyanate to ethyl chloroformate of 1-1.1 are adopted for further study in the orthogonal test.
3.4 Optimization of synthetic technology
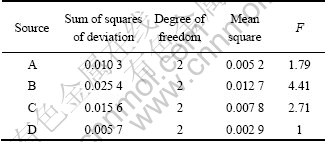
As discussed above, the content of catalyst, reaction temperature, reaction time and molar ratio of sodium thiocyanate to ethyl chloroformate are generally considered to be the important factors that affect the yield of ECIT. The investigated levels of each factor are selected depending on the above experiment results of the single-factor. Optimization of synthetic technology can be carried out by using an experimental design. The results of the orthogonal test and extreme difference analysis are presented in Table 2, and the results of variance analysis are listed in Table 3. According to the R value and the result of analysis of variance table, it can be found that the influence on the mean yields of ECIT decreases in the order of B>C>A>D. It can also be found that the reaction temperature is the most important determinant of the yield. So, the optimization of synthetic technology of ECIT is B3C3A3D2, i,e, reaction temperature is 35 ℃, reaction time is 3 h, the content of catalyst (molar fraction based on ethyl chloroformate) is 1.5% and molar ratio of sodium thiocyanate to ethyl chloroformate is 1.1, respectively. Under this synthetic technology, the yield can reach 96.8%. Elemental analysis results are as follows: measured value (theoretical value): C, 36.61% (36.59%); H, 3.83% (3.81%); N, 10.66% (10.67%). 1H NMR (DMSO-d6, TMS, δ): 4.16 (2H, q, CH2) and 2.30 (3H, t, CH3). IR spectral analysis results are as follows: 2 950 cm-1 (CH3), 2 866 cm-1 (CH2), 1 981 cm-1 and 865 cm-1 (N=C=S), 1 715 cm-1 (C=O) and 1 250 cm-1 (O—C). The measured data are consistent with literature values [16].
Table 2 Form of orthogonal test and experimental results
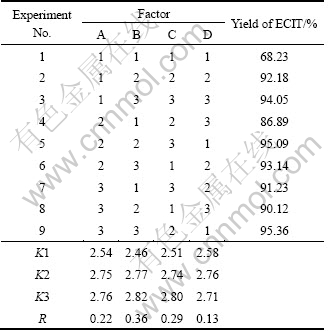
Table 3 Variance analysis for experimental results
4 Conclusions
1) Using Schiff base as a phase transfer catalyst, ethoxycarbonyl isothiocyanate is synthesized by reacting ethyl chloroformate with sodium thiocyanate. The whole reaction process has the advantages of simple operation, mild condition, and at the same time, the yield is high and the catalyst has good catalytic effect. It is suitable for industrial production of high efficient green synthesis technology.
2) The yield is influenced by the content of catalyst, reaction temperature, reaction time and the proportion of materials, and the reaction temperature is the most important determinant of the yield.
3) The optimum synthetic technology is determined using orthogonal test (L9(34)) as follows: reaction temperature 35 ℃, reaction time 3 h, the content of catalyst (molar fraction based on ethyl chloroformate) 1.5% and molar ratio of sodium thiocyanate to ethyl chloroformate 1.1. The experimental yield reaches 96.8% under the optimized synthetic technology.
References
[1] CHEN Wei-rong, SUN Nan, MO Wei-min. Preparation and applications of isothiocyanates [J]. Zhejiang Chemical Industry, 2009, 40(12): 21-25. (in Chinese)
[2] MI Li-xin, GAN Nan-qin, CHEEMA A, DAKSHANAMURTHY S, WANG Xian-tao, Yang DAVID C H, CHUNG Fung-lung. Cancer preventive isothiocyanates induce selective degradation of cellular a- and b-tubulins by proteasomes [J]. The Journal of Biological Chemistry, 2009, 284(25): 17039-17051.
[3] FIMOGNARI C, LENZI M, HRELIA P. Chemoprevention of cancer by isothiocyanates and anthocyanins: Mechanisms of action and structure-activity relationship [J]. Current Medicinal Chemistry, 2008, 15(5): 440-448.
[4] YUAN Lu, ZHONG Hong, LIU Guang-yi. Synthesis and application of isothiocyanates [J]. Fine Chemical Intermediates, 2007, 37(6): 10-13.
[5] DASH P K. Methods and compositions for treatment of central nervous system injury with isothiocyanates [P]. United States Patent, US0116423 A1, 2006-01-01.
[6] WESO?OWSKA A, GRO? L, WESTERLICH S, TADEUSZ S J. Synthesis and reactions of p-hydroxythiobenzamides [J]. Arkivoc, 2008(xv): 239-255.
[7] LEWELLYN M E, WANG S S, STRYDOM P J. Preparation of ethoxycarbonyl isothiocyanate using a pyridine or quinoline catalyst [J]. J Org Chem, 1990, 55(18): 5230-5231.
[8] FU Y L, STRYDOM P J. Process for the production of isothiocyanate derivatives [P]. United States Patent, US4659853, 1987-04-21.
[9] KULKANI S V, DESAI V C. Process for manufacture of N-alkoxy (or aryloxy) carbonyl isothiocyanate derivatives in the presence of N, N-dialkylarylamine catalyst and aqueous solvent [P]. United States Patent, US 6184412, 2001-02-06.
[10] XIA Jiang-bin, YANG Hong, LI Fu-you, HUANG Chun-hui. Synthesis of a novel complex of Schiff-base and zinc and application to sensitized nanocrystalline TiO2 solar cell [J]. Chemical Journal of Chinese Universities, 2006, 27(2): 204-207. (in Chinese)
[11] CHEN Yu-hong, TANG Zhi-yuan, TONG Ru-ting, WANG Qing-fei. Effect of Schiff bases structure on corrosion inhibition efficiency of copper [J]. Journal of Chinese Society for Corrosion and Protection, 2007, 27(3): 156-161. (in Chinese)
[12] DROZDZAK R, ALLAERT B, LEDOUX N, DRAGUTAN I, DRAGUTAN V, VERPOORT F. Synthesis of Schiff base-ruthenium complexes and their applications in catalytic processes [J]. Advanced Synthesis & Catalysis, 2005, 347(14): 1721-1743.
[13] GUPTA K C, SUTAR A K. Catalytic activities of Schiff base transition metal complexes [J] Coordination Chemistry Reviews, 2008, 252(12/13/14): 1420-1450.
[14] KUMAR S, DHAR D N, SAXENA P N. Applications of metal complexes of Schiff bases-A review [J]. Journal of Scientific and Industrial Research, 2009, 68(3): 181-187.
[15] ZHANG Qing-feng, JIANG Zi-tao, DONG Feng-guang, LI Rong. Titrimetric method for quantitative determination of the isothiocyanates with diethyl amine [J]. China Condiment, 2005, 2: 48-51. (in Chinese)
[16] LIU Hong-min. Practical spectral analysis for organic compounds [M]. Zhengzhou: Zhengzhou University Press, 2008. (in Chinese)
(Edited by YANG Bing)
Foundation item: Project(2007AA06Z122) supported by the National High Technology Research and Development Program of China; Project(20110491267) supported by the Postdoctoral Science Foundation of China; Project(74341015502) supported by Postdoctoral Fund of Central South University, China
Received date: 2011-08-24; Accepted date: 2011-10-31
Corresponding author: LIANG Wen-jie, PhD; Tel:+86-731-88879616; E-mail: liang_wenjie@163.com