Ti-Al体系含量对激光引燃自蔓延连接Cf/Al与TiAl接头显微组织和力学性能的影响
来源期刊:中国有色金属学报(英文版)2015年第5期
论文作者:冯广杰 李卓然 冯士诚 申忠科
文章页码:1468 - 1477
关键词:Cf/Al复合材料;TiAl合金;连接;自蔓延高温合成;中间层;Ti-Al含量
Key words:Cf/Al composite; TiAl alloys; joint; self-propagating high-temperature synthesis; interlayer; Ti-Al content
摘 要:采用Ni-Al-Ti作中间层,通过激光引燃自蔓延方法对Cf/Al复合材料与TiAl合金进行连接,研究中间层中Ti-Al含量对接头界面显微组织和力学性能的影响。接头中发生了母材的局部融化,并在两侧母材处生成了γ-Ni0.35Al0.30Ti0.35、NiAl3和Ni2Al3反应层。接头缺陷(如气孔、裂纹)降低了接头的致密度,并作为断裂源使接头强度急剧下降。添加Ti-Al可以改善接头致密度,并增强中间层产物与Cf/Al复合材料的附着力。通过改变Ti-Al含量,可以控制接头缺陷。当Ti-Al含量为0.1时,接头中无裂纹产生,致密度良好,强度达到最高值24.12 MPa.
Abstract: Cf/Al composites and TiAl alloys were joined by laser ignited self-propagating high-temperature synthesis (SHS) with Ni-Al-Ti interlayer. The effect of Ti-Al content on interfacial microstructure and mechanical properties of the joints was investigated. Localized melt of the substrates occurred in the joints. γ-Ni0.35Al0.30Ti0.35, NiAl3 and Ni2Al3 reaction layers formed adjacent to the substrates. Joint flaws, such as pores and cracks, made the joint density decrease and worked as the fracture source, which led to the sharp decline of joint strength. Additive Ti-Al increased joint density and strengthened the interlayer adhesion to Cf/Al. The joint flaws could be controlled by changing the Ti-Al content. When the Ti-Al content was 0.1, the joint was free of cracks with high density and reached the maximum shear strength of 24.12 MPa.
Trans. Nonferrous Met. Soc. China 25(2015) 1468-1477
Guang-jie FENG, Zhuo-ran LI, Shi-cheng FENG, Zhong-ke SHEN
State Key Laboratory of Advanced Welding and Joining, Harbin Institute of Technology, Harbin 150001, China
Received 13 May 2014; accepted 2 September 2014
Abstract: Cf/Al composites and TiAl alloys were joined by laser ignited self-propagating high-temperature synthesis (SHS) with Ni-Al-Ti interlayer. The effect of Ti-Al content on interfacial microstructure and mechanical properties of the joints was investigated. Localized melt of the substrates occurred in the joints. γ-Ni0.35Al0.30Ti0.35, NiAl3 and Ni2Al3 reaction layers formed adjacent to the substrates. Joint flaws, such as pores and cracks, made the joint density decrease and worked as the fracture source, which led to the sharp decline of joint strength. Additive Ti-Al increased joint density and strengthened the interlayer adhesion to Cf/Al. The joint flaws could be controlled by changing the Ti-Al content. When the Ti-Al content was 0.1, the joint was free of cracks with high density and reached the maximum shear strength of 24.12 MPa.
Key words: Cf/Al composite; TiAl alloys; joint; self-propagating high-temperature synthesis; interlayer; Ti-Al content
1 Introduction
As a new kind of structural materials, carbon fiber-reinforced aluminum matrix composites (Cf/Al) have low density and thermal expansivity, high specific strength and stiffness, excellent electrical and thermal conductivities, which make them successfully demonstrate their potential in aerospace and automotive applications [1-3]. Titanium aluminide (TiAl) is also characterized by its low density and high specific strength at elevated temperature [4-6]. The joining of Cf/Al composites and TiAl alloys allows us to take full advantage of their merits and further expand the scope of their applications.
However, owing to their notable differences in properties, especially for the temperature sensitivity and low melting point of Cf/Al composites compared with TiAl alloys, sound joint of Cf/Al composites and TiAl alloys cannot be obtained easily. High temperature, usually over 1073 K [7,8], was employed in the brazing of TiAl alloys, which was obviously inappropriate for the aluminum matrix composites. Other conventional welding processes, such as diffusion welding [9] and fusion welding [10], would also damage the connection between the reinforced fibers and matrix in composites and cause the formation of brittle Al4C3 in the joint. Thus, innovative welding method must be developed.
Self-propagating high-temperature synthesis (SHS) is a promising technique, and can be used to join dissimilar materials [11-13]. In the joining process, exothermic reaction occurs and compounds are in-situ synthesized. Then, materials are joined by the localized heat without thermal damage. Successes have been achieved in the joining of TiAl alloys and ceramics with Ti-Al-C by FENG et al [4,11] and WANG et al [14] and Ni-Al interlayer by PASCAL et al [13]. The ignition temperature of joining interlayer, such as Ti-Al-C and Ni-Al is close to the melting point of aluminum. So far, the SHS joining has not applied on the aluminum composites. And one important reason is that traditional ignition method, such as thermal explosion and arc ignition, cannot efficiently protect the low-melting aluminum composites when ignite the interlayer.
In this work, SHS joining was applied on the Cf/Al composites and TiAl alloys with Ni-Al-Ti interlayer. Laser beam was employed to ignite the interlayer for its small heating area and high heating rate. The typical interfacial microstructure of SHS joint was characterized. In addition, the effect of Ti-Al content on the joint microstructure and shear strength was systematically evaluated.
2 Experimental
The Cf/Al composites applied in this work had a fiber volume fraction of 50% and metal matrix of 6061 aluminum alloy. The Cf/Al composites were achieved through extrusion casting method, with a density of 2.189 g/cm3. TiAl alloys had the nominal composition Ti-48Al-7V-0.3Y (mole fraction, %). The Cf/Al and TiAl substrates were cut into 5 mm × 5 mm × 4 mm and 12 mm × 8 mm × 2 mm pieces, respectively. All the joined surfaces were polished by SiC papers up to grit 1000, and all the samples were cleaned ultrasonically in acetone for 15 min prior to the joining.
Powder mixtures of titanium (45 μm, 99.5%), aluminum (45 μm, 99.5%), and nickel (63 μm, 99.5%) were used and weighed out according to corresponding composition. The weighed powders were dry-mixed thoroughly in the tumbler ball mill using Al2O3 balls for 2 h, with the rotation speed of 300 r/min. The ball-to- powder mass ratio was 10:1. Then, the milled powders were cold pressed under the pressure of 200 MPa into disc specimens of 10 mm in diameter and 1 mm in thickness.
The joining process proceeded in the atmospheric environment. The green powder compact was sandwiched between the Cf/Al and TiAl substrates with a pressure of 2 MPa provided by special fixture. A laser beam of output power of 100 W and beam size of 0.2 mm heated the interlayer for 5 s and ignited the interlayer. Figure 1 shows the schematic diagram of the joining system.
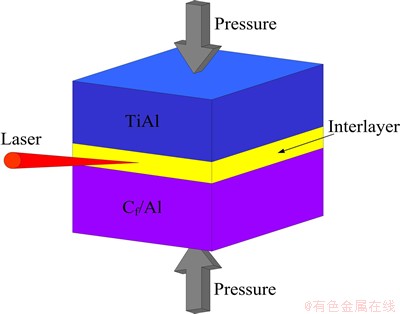
Fig. 1 Schematic diagram of joining system
After joining process, the polished cross-sections of the joints and reaction products were examined to characterize the microstructures by scanning electron microscopy (SEM) coupled with energy dispersive spectroscopy (EDS). The metallographic phases were identified by X-ray diffraction (XRD) (JDX-3530 M) with Cu Kα radiation operated at a voltage of 40 kV, working current of 40 mA and angle incidence of 10-90°. As shown in Fig. 2, room temperature shear test was conducted employing an Instron-1186 universal testing machine by a specially designed fixture at a crosshead rate of 0.5 mm/min and the average strength of three joints joined under the same conditions was used.
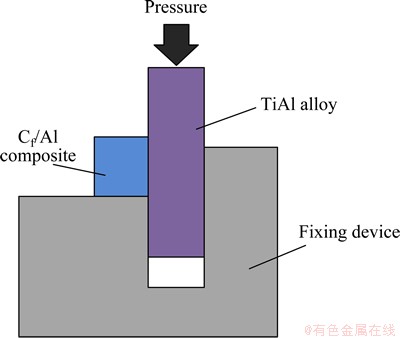
Fig. 2 Schematic diagram of shear test of joint
3 Results and discussion
3.1 Selection of interlayer
In SHS joining, the interlayer served as heat source and filler metal. Thus, it determined the joining quality that whether the interlayer had high exothermicity and the reaction products were homogeneous with the substrates in physical and chemical properties. Owing to the particular heat transfer condition, few reactant powders for fabricating materials can meet the requirement. The Ti-Al-C interlayer was regarded as the first choice in SHS joining. According to previous studies [11,15], the ignition temperature of Ti-Al-C interlayer was close to the melting point of aluminum. Its exothermic reaction consisted of two stages. The first stage is that Ti reacts with Al and a small quantity of heat is released, then the reaction of Ti and C is induced by the first stage and large quantity of heat is released. In this work, aluminum composites limited the integral heating of thermal explosion. When the Ti-Al-C interlayer was ignited in other ways, the reaction of Ti and C in the second stage cannot be induced owing to the cooling effect of adjacent substrates. Only a certain amount of heat was produced, which would lead to the weak bonding. Clearly, interlayer with high and rapid exothermicity was needed.
According to the Ni-Al phase diagram, compounds, such as NiAl3, Ni2Al3, NiAl, can be formed between Ni and Al. The addition of Ni allowed the formation of reaction layers between the interlayer and substrates, which were the key to interfacial bond formation. When the powder mixture of Ni and Al was employed, enough joining heat could be provided by the sharp exothermic reaction. In addition, a given amount of Ti should also be contained to relieve the chemical mismatch between the reaction products and substrates. Thus, the interlayer was determined to be a mixture of Ni, Al and Ti powders.
The reaction in the Ni-Al-Ti powders was designed as below:
Ni+(m+1)Al+mTi=NiAl+mTiAl (1)
where m represents the content of Ti-Al in the products.
Adiabatic combustion temperature (Tad), which means the maximum combustion temperature achieved under adiabatic condition, is the most important thermodynamic parameter to characterize the SHS reaction. The Tad can be calculated through following formula:
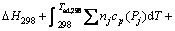
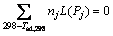 (2)
(2)
where Pj and nj respectively refer to the products and the stoichiometric coefficients of products. cp(Pj) and L(Pj) are the specific heat capacity and phase transformation enthalpy (if a phase change occurs in the products) of the products, respectively.
Tad of the Ni-Al-Ti interlayer was calculated in different reactant compositions, as shown in Fig. 3. Owing to the low exothermicity of TiAl compared with NiAl, TiAl worked as the coolant in the reaction. The curve showed lowering trend with the increase of Ti-Al content. When the coefficient m was 1.66, Tad reached 1800 K.

Fig. 3 Adiabatic temperature of Ni-Al-Ti interlayer with different Ti-Al contents
According to the experiential criterion by MERZHANOV et al [16,17], a reaction can be self-sustaining only when Tad≥1800 K. In SHS joining, self-sustaining reaction is the minimum requirement. A sound joint must be dense, in which the pores in the reactants must be discharged in joining process. The best approach is finding an interlayer whose Tad is equal or higher than the melting point of the product. When the product is molten, it can easily be densified under joining pressure. The melting point of NiAl is 1911 K, which is equal to its adiabatic temperature. The additive Ti-Al decreased the Tad, which meant that the adiabatic temperature of Ni-Al-Ti interlayer could never reach 1911 K. However, when the Ti-Al content was low, heat from laser beam could offset the temperature difference. Thus, the Ti-Al content of interlayer should be as low as possible under the premise of the physical and chemical homogeneity with substrates.
3.2 Typical microstructure of laser ignited SHS joint
Sound joint of Cf/Al and TiAl with the 63.0Ni-31.9Al-5.1Ti (mass fraction, %) interlayer, which corresponded to the coefficient m of 0.1, was obtained by laser ignited SHS joining. Figure 4 shows the typical joint microstructure. As shown in Fig. 4, the Ni-Al-Ti interlayer reacted completely without any residual Ni, Al, Ti particles, and the joint was free of cracks and obvious pores. Due to the particularity of SHS joining, very small amount of voids were unavoidable.
Adjacent to TiAl substrate, a reaction layer with a thickness of 3-4 μm (noted as Layer I) formed. meanwhile, some white phases distributed in it. Near Layer I, a mixture of offwhite, lightgray, gray and dark gray phases generated. On the other side, the Cf/Al reacted to the interlayer and formed two reaction layers (Layer II and Layer III), which both had a thickness of 4-5 μm. Furthermore, Layer III extended into the composites and surrounded some light gray phase, which was similar to Layer II. Meanwhile, an eutectic structure was observed in the middle of the joint.
To identify the possible phases presented in the joint, EDS compositional analyses were conducted. According to the EDS results in Table 1, Layer I primarily contained Ni, Al, Ti (Point A). Based on the XRD analyses results of the fracture section on Layer I (Fig. 5(a)), Layer I was confirmed to be γ-Ni0.35Al0.30Ti0.35, which was also approved in Refs. [18-20]. The white phase (Point B) contained much Y and Al, and was regarded as the YAlx from the TiAl substrate. In Fig. 4(b), the offwhite phase (Point C), lightgray phase (Point D), and gray phase (Point E) mainly consisted of Ni and Al. Among them, the n(Ni)/n(Al) values of offwhite phase (Point C) and gray phase (Point E) were approximately 3:1 and 1:1, and the phases were supposed to be Ni3Al and NiAl, respectively.
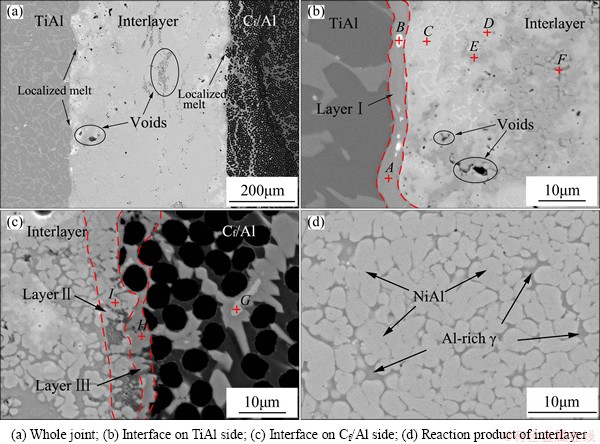
Fig. 4 SEM images of joint
Table 1 EDS compositional analyses result of joint
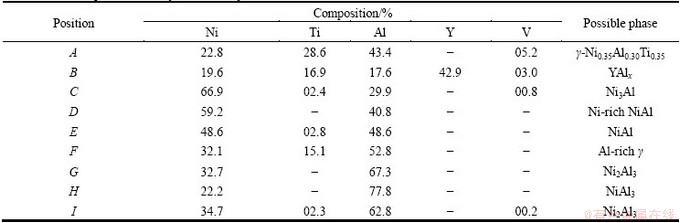
The lightgray phase (Point D) had an approximate n(Ni)/n(Al) value of 5:3, which was the same with that of Ni5Al3. However, the very low free energy change associated with the formation of Ni5Al3 makes the nucleation factually impossible in Ni-Al diffusion couples [21]. Thus, the Ni5Al3 can only be obtained by quenching NiAl of the corresponding composition and aging the resultant martensite at T<973 K [22,23], which is totally different with the SHS joining condition. According to the Ni-Al phase diagram, the content of Ni in NiAl ranges from 45% to 60%, and is even higher at high temperature. Due to the instantaneous reaction of SHS joining, atoms of partial NiAl phase at high temperature did not have much time to homogenize, and the high content of Ni atoms might be kept in the cooling process. Thus, the lightgray phase was supposed to be Ni-rich NiAl.
The eutectic microstructure in Fig. 4(d) consisted of gray NiAl phase (Point E) and dark gray phase (Point F), which were confirmed as NiAl and γ-Ni0.35Al0.30Ti0.35 through XRD analyses (Fig. 5(b)). The dark gray phase (Point F) was taken for Al-rich γ phase due to the high Al content compared with γ-Ni0.35Al0.30Ti0.35. On the Cf/Al side, the interlayer reacted with the aluminum matrix, and NiAl3 layer (Point H in layer III) and Ni2Al3 layer (Point I in layer II) formed. Furthermore, Ni atoms diffused from the interlayer to the Cf/Al. The NiAl3 layer extended into the Cf/Al and was surrounded by some Ni2Al3 phases.
Furthermore, one noteworthy phenomenon was that the interfaces of laser ignited SHS joint were rugged contrast with brazing and diffusion joints [6-8,24,25]. According to the thermodynamic calculation, Tad of the applied interlayer was calculated to be 1894.82 K. Under the effect of laser beam and high exothermic reaction, the substrates locally melted and the rugged interfaces formed. It was because the localized melt made the sufficient atom diffusion between the interlayer and substrates during the short time. Then, γ-Ni0.35Al0.30Ti0.35, Ni2Al3 and NiAl3 layers produced and the joint took shape. Simultaneously, more liquid phase would emerge on the Cf/Al side owing to the lower melting point of aluminum matrix (933 K), which made the diffusion of Ni show a strong tendency in the Cf/Al.
3.3 Effect of Ti-Al content on joint microstructure
According to Eq. (1), the interlayer product was designed to be NiAl+mTiAl. To study the effect of Ti-Al content on joint microstructure, different values of coefficient m were set. Figure 6 provides the morphology of Cf/Al/TiAl joints with four interlayer compositions of 0, 0.05, 0.1 and 0.2 Ti-Al content, respectively. With different Ti-Al contents, the joint microstructure had some significant changes. The most visible change in Fig. 6 was the joint density. When the coefficient m was 0, which meant that in the Ni-Al interlayer, large amount of pores existed in the interlayer product and the joint density was low. Meanwhile, some white inhomogeneous Ni3Al and Ni2Al3 phases formed on two sides. With the increase of Ti-Al content, the amount and size of pores decreased and the joint density had a remarkable increase. The joint was dense when the coefficient m was 0.1, and the further increase of Ti-Al content had a small effect on joint density.
Figure 6 also shows the microstructures of interlayer products with four compositions, and regular transformation occurred on the interlayer products. When the Ti-Al content was 0, the product was single NiAl phase. With the increase of Ti-Al content, the products transformed into an eutectic microstructure of NiAl+Al-rich γ. Nevertheless, some blocky Ni-rich NiAl still existed owing to the poverty of Ti when the coefficient m was 0.05. Then, the Ni-rich NiAl disappeared on the composition of NiAl+0.1TiAl. The further increase of Ti-Al content had no effect on the type of interlayer product, and only caused the rise of Al-rich γ content.
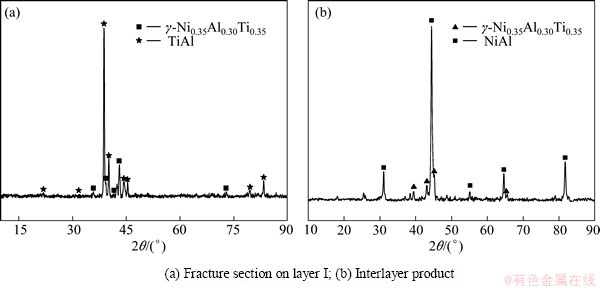
Fig. 5 XRD patterns of joint
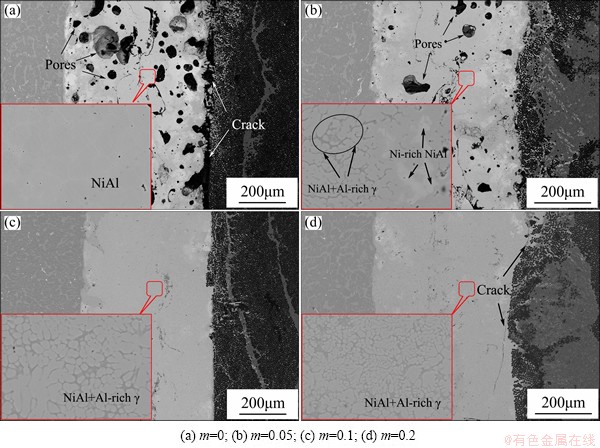
Fig. 6 Effect of Ti-Al content on joint microstructure
Compared with single NiAl phase, the eutectic microstructure of NiAl+Al-rich γ had a lower melting point. Under the effect of joining heat, the formation of eutectic microstructure could lengthen the molten product existing time. It was all owing to the molten state that the pores could be easily exhausted and the joint density increased, which was obvious in the contrast shown in Fig. 6. Ti-Al content also affected the joint interface, as shown in Fig. 7. The additive Ti-Al worked as the coolant in the reaction. Thus, the Tad showed a lowering trend with the increase of Ti-Al content, as shown in Fig. 3. When the Ti-Al content was 0, the interlayer had the violentest reaction, and many voids formed in the γ-Ni0.35Al0.30Ti0.35 layer. In addition, a coefficient of thermal expansion (CTE) mismatch existed between the TiAl substrate (about 10.1×10-6 K-1) and the reaction product (about 15×10-6 K-1). Under the rapid heating and cooling conditions, high thermal stress at the interface caused the appearance of microscopic cracks. With the increase of Ti-Al content, the reaction violence moderated and the thermal stress decreased gradually. Defects such as pores and cracks disappeared in the γ-Ni0.35Al0.30Ti0.35 layer. However, when the TiAl content was 0.2, the low joining heat led to the rapid drop of the γ-Ni0.35Al0.30Ti0.35 layer thickness and weakened the bonding between the interlayer and TiAl substrate. Continuous cracks formed in the reaction layer and propagated to the interlayer product, as shown in Fig. 7(d).
On the Cf/Al side, the composites locally melted due to the large heat released in the joining. According to the phase diagram, no compounds can form between Ni and C. On the contrary, the ΔGΘ of Ni-Al reaction (Ni+Al=NiAl, ΔGΘ=-108.524 kJ/mol; 2Ni+3Al=Ni2Al3, ΔGΘ=-267.738 kJ/mol; 3Ni+Al=Ni3Al, ΔGΘ=-139.967 kJ/mol) has a really large negative value, which means the spontaneous reaction. When the interlayer was Ni-Al, the localized molten aluminum matrix reacted with the Ni. NiAl3 layer formed and extended into the Cf/Al. Aluminum matrix was excessively consumed. The carbon fiber had a melting point over 3000 K and remained in the solid state during the joining. The NiAl3 layer had a very low adhesion on the carbon fiber. Thus, a significant crack produced on the composites side, as shown in Fig. 8(a). Owing to the strong atom attraction of Ti and C, the active element Ti of additive Ti-Al could increase the adhesion of the interlayer product on the carbon fibers. As shown in Figs. 8(a)-(c), the bonding of interlayer product and the composites had significant improvement with the increase of Ti-Al content. The crack became discontinuous gradually until disappeared. When the Ti-Al content was 0.2, the eutectic microstructure extended into the aluminum matrix. Microscopic crack came out again owing to the mismatch of their CTE (about transverse 7.56×10-6 K-1 and longitudinal 0.27×10-6 K-1 of Cf/Al, about 15×10-6 K-1 of reaction product).
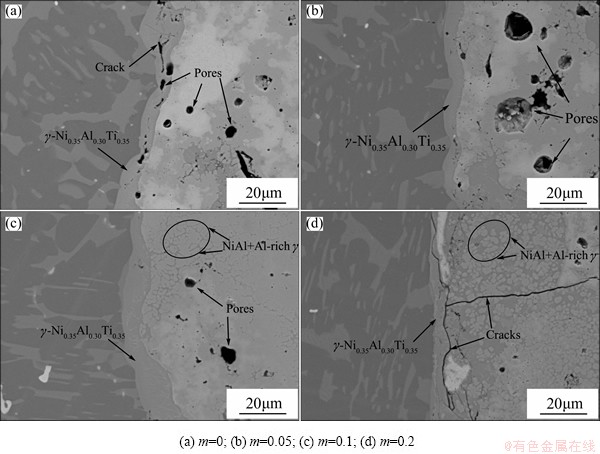
Fig. 7 Effect of Ti-Al content on TiAl interface
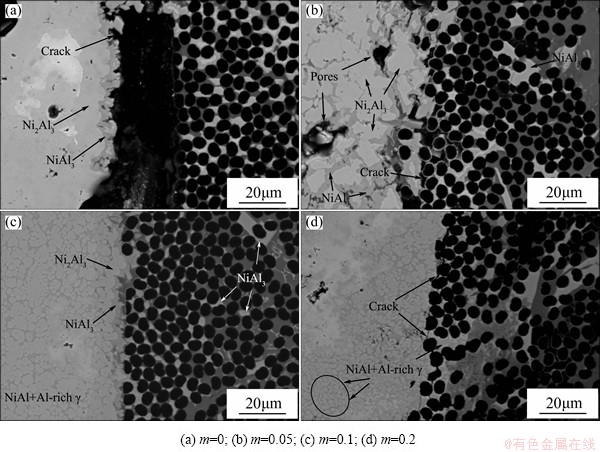
Fig. 8 Effect of Ti-Al content on Cf/Al interface
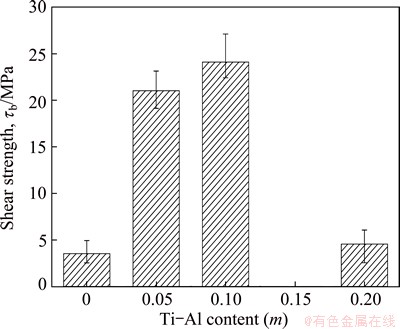
Fig. 9 Effect of Ti-Al content on shear strength of joint
3.4 Mechanical properties of Cf/Al/TiAl joint
Figure 9 illustrates the shear strength of Cf/Al/TiAl joint with different Ti-Al contents. The maximum shear strength of 24.12 MPa was obtained when the Ti-Al content was 0.1. In a microstructural view, the shear strength is largely dependent on the joint microstructure. The joint microstructure had a noteworthy change both in reaction products and joint flaws. When Ti-Al content was 0, sharp reaction occurred in Ni-Al interlayer, and caused large amount of pores in the joint. On the Cf/Al side, the interlayer reacted with aluminum matrix and formed the NiAl3 and Ni2Al3 layers. A huge and continuous crack formed because of the excessive consumption of aluminum matrix and the low adhesion of NiAl3 layer on carbon fibers. The fracture of test samples occurred on the Cf/Al side, as shown in Fig. 10(a). The joint only had a poor strength of 3.56 MPa. As Ti-Al content increased, the interlayer product transformed from NiAl to the eutectic organization of NiAl+Al rich-γ. The formation of eutectic organization restrained the existence of pores and cracks. Meanwhile, the adhesion of the interlayer product on the composites was enhanced by the addition of active Ti. Joint flaws had an obvious reduction, as shown in Fig. 6(b). Thus, the shear strength had a significant elevation when Ti-Al content was 0.05. Desired interfacial microstructure was obtained when Ti-Al content was 0.1. Mechanics performance improvements stemmed from the high joint density and absence of cracks. Additionally, appropriate heat releasing relieved the interface thermal stress and produced suitable thickness of reaction layers on both sides, which made the joint shear strength reach a maximum. The test samples with Ti-Al content of 0.1 usually cracked along the reaction layers on both sides and crossed the reaction product, demonstrating a brittle fracture as shown in Fig. 11. The further increase of Ti-Al content to 0.2 led to the thinner γ-Ni0.35Al0.30Ti0.35 layer on TiAl side and the extension of eutectic microstructure into composites. Cracks appeared again under the effect of thermal stress, which caused a sharp drop of the shear strength to 4.58 MPa. Figure 10(b) shows the fracture place after shear test, and the path ran through the whole joint.
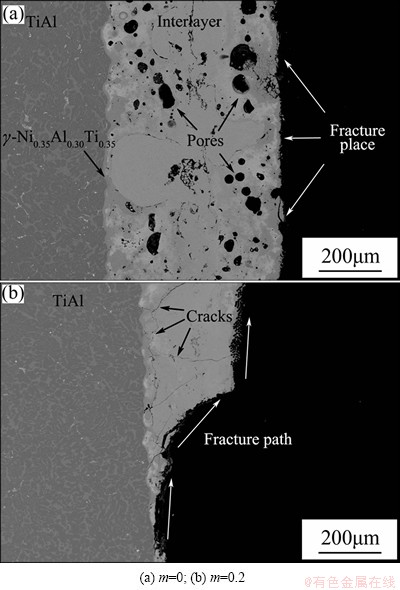
Fig. 10 Joints fracture places with different Ti-Al contents
Figure 11 shows the fracture morphology of joint with Ti-Al content of 0.1. Based on the EDS results, the fracture mainly happened in Cf/Al substrate and γ-Ni0.35Al0.30Ti0.35 layer, and crossed the interlayer product. According to Fig. 4(c), the NiAl3 layer extended into the composites. Brittle NiAl3 decreased the deformation ability of aluminum matrix, damaged the integrity of Cf/Al and showed some brittle features. The carbon fibers were stripped from the matrix and some cracked NiAl3 distributed on the surface, as shown in Fig. 11(b). In addition, the rugged interface of NiAl3 layer (Fig. 4(c)) would change the crack propagating direction. The crack propagated towards the joint center and crossed the interlayer product (Fig. 11(c)). Figure 11(d) shows the fracture surface of γ-Ni0.35Al0.30Ti0.35 layer on other side, and demonstrated a typical brittle characteristic with cleavage steps.
As can be seen, pores and cracks decreased the joint density and worked as the fracture sources, and affected the joint property. Through changing the Ti-Al content, these joint flaws could be controlled. In SHS joining, the generation of intermetallics ensured the rapid heat releasing to form the bonding. However, the intermetallics had greater brittleness and created the high joint residual stress together with large heating and cooling rate. This was also the reason why even the shear strength of sound joint was not very high. Though the room temperature strength of this joint was low, the joint could have good performance at high temperature owing to the formation of high-melting intermetallics.
4 Conclusions
1) The joining interlayer was chosen to be the Ni-Al-Ti system in consideration of high exothermicity and chemical compatibility with the substrates. Thermo-dynamic calculation was conducted and adiabatic temperature was calculated with different Ti-Al contents. The adiabatic temperature showed a lowering trend with the increase of Ti-Al content.
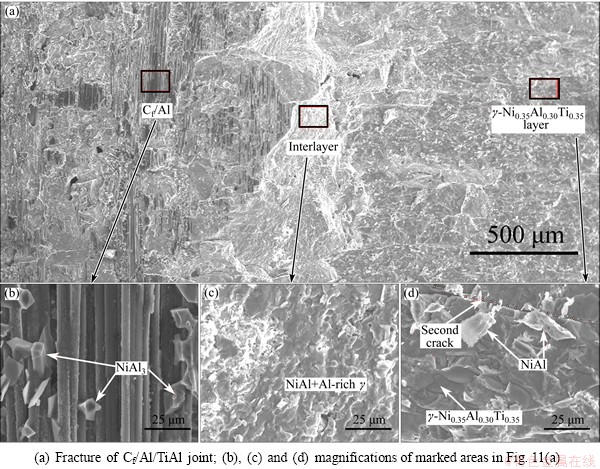
Fig. 11 Typical fractural surfaces of Cf/Al/TiAl joint with Ti-Al content of 0.1
2) Sound SHS joint of Cf/Al composites and TiAl alloys was obtained with Ni-Al-Ti interlayer. Localized melt occurred on the substrates under the effect of sharp SHS reaction and stimulated the interfacial bond formation. Interlayer product was an eutectic microstructure of NiAl+Al-rich γ. γ-Ni0.35Al0.30Ti0.35 and NiAl3, Ni2Al3 reaction layers, respectively, formed in the TiAl/interlayer and Cf/Al/interlayer interfaces.
3) Pores and cracks appeared in the joint when the Ti-Al content was 0. These flaws decreased the joint density and worked as the fracture sources, leading to the low joint strength. Additive Ti-Al made interlayer product transform from NiAl to the eutectic microstructure of NiAl+Al-rich γ, which increased the joint density and the adhesion on Cf/Al. Interfacial microstructure and joint flaws could be controlled through changing the Ti-Al content. The cracks disappeared and then reappeared with increasing Ti-Al content, made the joint strength firstly increase and then decrease. When the Ti-Al content was 0.1, the joint was free of cracks with high density, reached the maximum strength of 24.12 MPa.
References
[1] LI D G, CHEN G Q, JIANG L T, XIU Z Y, ZHANG Y H, WU G H. Effect of thermal cycling on the mechanical properties of Cf/Al composites [J]. Materials Science and Engineering A, 2013, 586: 330-337.
[2] DAOUD A. Microstructure and tensile properties of 2014 Al alloy reinforced with continuous carbon fibers manufactured by gas pressure infiltration [J]. Materials Science and Engineering A, 2005, 391: 114-120.
[3] MA Y Q, QI L H, ZHENG W Q, ZHOU J M, JU L Y. Effect of specific pressure on fabrication of 2D-Cf/Al composite by vacuum and pressure infiltration [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 1915-1921.
[4] CAO J, FENG J C, LI Z R. Effect of reaction heat on reactive joining of TiAl intermetallics using Ti-Al-C interlayers [J]. Scripta Materialia, 2007, 57: 421-424.
[5] SHU S L, QIU F, XING B, JIN S B, WANG J G. Effect of strain rate on the compression behavior of TiAl and TiAl-2Mn alloys fabricated by combustion synthesis and hot press consolidation [J]. Intermetallics, 2013, 43: 24-28.
[6] LI J B, LIU Y, WANG Y, LIU B, LU B, LIANG X P. Constitutive equation and processing map for hot compressed as-cast Ti-43Al-4Nb-1.4W-0.6B alloy [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3383-3391.
[7] LI Z R, FENG J C, CAO J. Vacuum diffusion bonding of TiB2 to TiAl based alloys [J]. Materials Science and Technology, 2004, 20: 1666-1668.
[8] SONG X G, CAO J, CHEN H Y, WANG Y F, FENG J C. Brazing TiAl intermetallics using TiNi-V eutectic brazing alloy [J]. Materials Science and Engineering A, 2012, 551: 133-139.
[9] SUZUMURA A, XING Y J. Diffusion brazing of short Al2O3 fiber-reinforced aluminum composite [J]. Materials Transactions, JIM, 1996, 37: 1109-1115.
[10] KENNDY J R. Microstructural observations of arc welded boron-aluminum composites [J]. Welding Journal, 1973, 3: 120-124.
[11] FENG J C, CAO J, LI Z R. Microstructure evolution and reaction mechanism during reactive joining of TiAl intermetallic to TiC cermet using Ti-Al-C-Ni interlayer [J]. Journal of Alloys and Compounds, 2007, 436: 298-302.
[12] ROSA R, VERONESI P, HAN S, CASALEGNO V, SALVO M, COLOMBINI E, LEONELLI C, FERRARIS M. Microwave assisted combustion synthesis in the system Ti-Si-C for the joining of SiC: Experimental and numerical simulation results [J]. Journal of the European Ceramic Society. 2013, 33: 1707-1719.
[13] PASCAL C, MARIN-AYRAL R M,  J C. Joining of nickel monoaluminide to a superalloy substrate by high pressure self-propagating high-temperature synthesis [J]. Journal of Alloys and Compounds, 2002, 337: 221-225.
J C. Joining of nickel monoaluminide to a superalloy substrate by high pressure self-propagating high-temperature synthesis [J]. Journal of Alloys and Compounds, 2002, 337: 221-225.
[14] WANG J H, CHENG J, BAI P K, LI Y X. Investigation of joining Al-C-Ti cermets and Ti6Al4V by combustion synthesis [J]. Materials Science and Engineering B, 2012, 177: 1703-1706.
[15] RAPP R A, ZHENG X J. Thermodynamic consideration of grain refinement of aluminum alloys by titanium and carbon [J]. Metallurgical and Materials Transactions A, 1991, 22: 3071-3075.
[16] MERZHANOV A G, BOROVINSKAYA I P. Self-propagating high- temperature synthesis of inorganic compounds [J]. Doklady Akademii Nauk SSSR, 1972, 204(2): 429-432.
[17] MERZHANOV A G. Problem of combustion process in chemical technology and metallurgy [J]. Russian Chemical Reviews, 1976, 45(5): 827-848.
[18] CHENG Y X, WANG W, ZHU S L, XIN L, WANG F H. Arc ion plated-Cr2O3 intermediate film as a diffusion barrier between NiCrAlY and γ-TiAl [J]. Intermetallics, 2010, 18: 736-739.
[19] KOPIT Y. The ability of systems based on Ni, Al and Ti to be synthesized by self-propagating high-temperature synthesis (SHS) [J]. Intermetallics, 2001, 9: 387-393.
[20] PENG L M. Fabrication and mechanical properties of microalloyed and ceramic particulate reinforced NiAl-based alloys [J]. Journal of Alloys and Compounds, 2007, 440: 150-153.
[21] FARBER L, GOTMAN I, GUTMANAS E Y. Formation of Ni5Al3 in Ni-Al laminated structures and powder blends [J]. Materials Letters, 1998, 34: 226-231.
[22] ENAMI K, NENNO S. A new ordered phase in tempered 63.8Ni-1Co-Al martensite [J]. Transactions of the Japan Institute of Metals, 1978, 19: 571-580.
[23] ROBERTSON I M, WAYMAN C M. Ni5Al3 and the nickel-aluminum binary phase diagram [J]. Metallography, 1984, 17: 43-55.
[24] DONG H G, YANG Z L, YANG G S, DONG C. Vacuum brazing of TiAl alloy to 40Cr steel with Ti60Ni22Cu10Zr8 alloy foil as filler metal [J]. Materials Science and Engineering A, 2013, 561: 252-258.
[25] WANG H Q, CAO J, FENG J C. Brazing mechanism and infiltration strengthening of CC composites to TiAl alloys joint [J]. Scripta Materialia, 2010, 63: 859-862.
冯广杰,李卓然,冯士诚,申忠科
哈尔滨工业大学 先进焊接与连接国家重点实验室,哈尔滨 150001
摘 要:采用Ni-Al-Ti作中间层,通过激光引燃自蔓延方法对Cf/Al复合材料与TiAl合金进行连接,研究中间层中Ti-Al含量对接头界面显微组织和力学性能的影响。接头中发生了母材的局部融化,并在两侧母材处生成了γ-Ni0.35Al0.30Ti0.35、NiAl3和Ni2Al3反应层。接头缺陷(如气孔、裂纹)降低了接头的致密度,并作为断裂源使接头强度急剧下降。添加Ti-Al可以改善接头致密度,并增强中间层产物与Cf/Al复合材料的附着力。通过改变Ti-Al含量,可以控制接头缺陷。当Ti-Al含量为0.1时,接头中无裂纹产生,致密度良好,强度达到最高值24.12 MPa.
关键词:Cf/Al复合材料;TiAl合金;连接;自蔓延高温合成;中间层;Ti-Al含量
(Edited by Yun-bin HE)
Foundation item: Project (51075101) supported by the National Natural Science Foundation of China
Corresponding author: Zhuo-ran LI; Tel: +86-451-86418038; E-mail: lizr@hit.edu.cn
DOI: 10.1016/S1003-6326(15)63747-5

