Superior Au-adsorption performance of aminothiourea-modified waste cellulosic biomass
来源期刊:中南大学学报(英文版)2018年第12期
论文作者:赵君梅 王福春 王万坤 刘会洲
文章页码:2992 - 3003
Key words:adsorption; reduction-deposition; waste cellulosic biomass; aminothiourea; gold nanoparticles
Abstract: Waste cellulosic biomass obtains various applications due to low-cost and eco-benign characteristics. A general strategy is proposed for waste cellulosic biomass to be modified with dialdehyde functional groups as intermediates through periodate partial oxidation. Finally, aminothiourea-modified waste cellulosic biomass can be prepared through Schiff reaction. Waste corn stalk, cotton and paper as typical precursors, were used to prepare cellulosic biomass, abbreviated as AT-S, AT-C and AT-P, respectively, and their adsorption behaviors of Au(III) from the hydrochloric acid medium were investigated. The pseudo-second kinetics equation as well as the Langmuir isotherm equation can be used to depict the adsorption process, and the maximum adsorption capacities of Au(III) are 21.4, 19.0 and 3.28 mol/kg for AT-S, AT-C and AT-P at 298 K, respectively. The adsorption capacities of Au(III) on aminothiourea modified corn stalk (AT-S) is almost 357 times greater than that of raw corn stalk. To the best of our knowledge, AT-S has the highest adsorption capacity towards Au(III). AT-S also displays a superior separation selectivity towards Au(III) in the presence of Cu(II), Ni(II), Co(II), Pt(VI), Pd(II) and Rh(III). Furthermore, the characterization analysis of XRD, TG, SEM, TEM and FTIR confirms that AuCl4– has been reduced to elemental Au nanoparticles and deposit onto the surface of the biomass. It shows a prospect for waste corn stalk to be used to adsorb Au(III) from liquid phase and the possible fabrication of gold nanoparticles by a general adsorption process without any reductant.
Cite this article as: WANG Fu-chun, ZHAO Jun-mei, WANG Wan-kun, LIU Hui-zhou. Superior Au-adsorption performance of aminothiourea-modified waste cellulosic biomass [J]. Journal of Central South University, 2018, 25(12): 2992–3003. DOI: https://doi.org/10.1007/s11771-018-3969-3.

J. Cent. South Univ. (2018) 25: 2992-3003
DOI: https://doi.org/10.1007/s11771-018-3969-3
WANG Fu-chun(王福春)1, ZHAO Jun-mei(赵君梅)2, WANG Wan-kun(王万坤)1, LIU Hui-zhou(刘会洲)2
1. School of Materials and Metallurgical Engineering, Guizhou Institute of Technology,Guiyang 550003, China;
2. Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: Waste cellulosic biomass obtains various applications due to low-cost and eco-benign characteristics. A general strategy is proposed for waste cellulosic biomass to be modified with dialdehyde functional groups as intermediates through periodate partial oxidation. Finally, aminothiourea-modified waste cellulosic biomass can be prepared through Schiff reaction. Waste corn stalk, cotton and paper as typical precursors, were used to prepare cellulosic biomass, abbreviated as AT-S, AT-C and AT-P, respectively, and their adsorption behaviors of Au(III) from the hydrochloric acid medium were investigated. The pseudo-second kinetics equation as well as the Langmuir isotherm equation can be used to depict the adsorption process, and the maximum adsorption capacities of Au(III) are 21.4, 19.0 and 3.28 mol/kg for AT-S, AT-C and AT-P at 298 K, respectively. The adsorption capacities of Au(III) on aminothiourea modified corn stalk (AT-S) is almost 357 times greater than that of raw corn stalk. To the best of our knowledge, AT-S has the highest adsorption capacity towards Au(III). AT-S also displays a superior separation selectivity towards Au(III) in the presence of Cu(II), Ni(II), Co(II), Pt(VI), Pd(II) and Rh(III). Furthermore, the characterization analysis of XRD, TG, SEM, TEM and FTIR confirms that AuCl4– has been reduced to elemental Au nanoparticles and deposit onto the surface of the biomass. It shows a prospect for waste corn stalk to be used to adsorb Au(III) from liquid phase and the possible fabrication of gold nanoparticles by a general adsorption process without any reductant.
Key words: adsorption; reduction-deposition; waste cellulosic biomass; aminothiourea; gold nanoparticles
Cite this article as: WANG Fu-chun, ZHAO Jun-mei, WANG Wan-kun, LIU Hui-zhou. Superior Au-adsorption performance of aminothiourea-modified waste cellulosic biomass [J]. Journal of Central South University, 2018, 25(12): 2992–3003. DOI: https://doi.org/10.1007/s11771-018-3969-3.
1 Introduction
Gold (Au) is widely used in various fields, such as medicine, electronics and jewelry. A large amount of Au-containing waste materials is recovered from manufacturing residues and disposed devices. The content of Au in the wastes is always higher than that in the Au ores. Particularly, some industrial waste streams, such as the refining or mining effluents containing low concentration of Au ions, have been regarded as the important second resources. Hence, it makes sense if Au could be recovered from these various wastes economically. To recover Au, leaching solid Au into liquid phase firstly and then through hydrometallurgical process is more economical. Various techniques have been developed including precipitation, adsorption, and solvent extraction, etc to recover Au from leach liquor [1]. Adsorption is obtaining more and more attention because it is highly efficient, environmentally benign without using any organic solvents, and especially suitable for recovery and/or removing of metal ions in dilute solutions [2–4]. Among them, traditional resin adsorption and active carbon adsorption are the two main methods to be used in industry [1]. However, the adsorption selectivity and capacity is not satisfying, especially it is not so low-cost and eco-friendly for the used adsorbents.
The recovery of Au via biomass is more and more attractive [1]. It has been reported that various pure polysaccharides treated by concentrated sulfuric acid can selectively adsorb Au(III) from chloride media with good adsorption capacities [5]. GURUNG et al [6] reported N-aminoguanidine- modified cellulose for selective biosorption of Au(III), and its adsorption capacities can be improved to be 9.2 mol/kg. In fact, it should be a more significant strategy to recover Au with waste biomass based adsorbents. Various modified bio-wastes have been extensively reported to adsorb Au(III), such as grape waste [7], persimmon waste [8, 9], and spent buckwheat hulls [10]. Concentrated sulfuric acid-treated waste paper [11] or cotton [12] has also been used to adsorb Au(III). The adsorption capacity of Au(III) on these bio-wastes, nevertheless, has not been highlighted.
Waste cellulosic biomasses have the characteristics of eco-benignity and university. They are usually composed of cellulose, hemicellulose and lignin, and their typical molecular structures are shown in Figure 1. We know that thiourea can efficiently complex with Au(III). It had been reported that thiourea groups [13–15] were fixed on chemical resins and used for the adsorption of noble metal ions. However, there are few reports about the modification of thiourea groups onto cellulosic biomasses to be used as adsorbent for Au(III). According to Ref. [16], cellulose or hemicellulose in cellulosic biomasses always contains a large quantity of adjacent hydroxyls. Theoretically, each pair of adjacent hydroxyls can be oxidized into one pair of dialdehydes selectively by periodate. Then, dialdehyde groups in the cellulosic bio-wastes can be modified facilely by various functional groups through Schiff reaction. Thus, aminothiourea can be immobilized onto cellulose through Schiff reaction between dialdehyde and aminothiourea, as shown in Figure 2.
In this work, as typical cellulosic biomasses, waste corn stalk, cotton and paper were used to prepare adsorbent through modification with thiourea groups, and their adsorption behaviors of Au(III) have been systematically studied. It can be expected that aminothiourea groups modified cellulosic bio-wastes can afford excellent adsorption abilities towards Au(III).
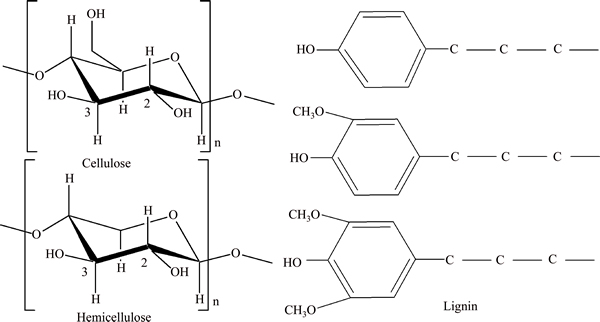
Figure 1 Typical molecular structure of cellulose, hemicellulose and lignin

Figure 2 Proposed mechanisms for celullose and hemicellulose modification
2 Materials and methods
2.1 Materials and apparatus
Quantitative filter paper (100% cellulose, mass fraction), cotton (100% cellulose), and corn stalk (major constituents: lignin 20.5%, hemicellulose 24.7% and cellulose 35.5%) were first dried and smashed to powder for use. Copper, cobalt, nickel, rhodium and palladium chlorides, HAuCl4·4H2O, H2PtCl6·6H2O and all other reagents were used as received.
Fourier transform infrared spectroscopy (FT-IR) (Bruker Tensor 27, Germany), digital pH meter (HANNA pH211, Italy), inductively coupled plasma-optical emission spectrometer (ICP-OES) (PekinElmer OPTIMA 7000DV, America), thermo gravimetric analyses (TG) (Netzsch Iris TG 209 C, Germany), scanning electronic microscope (SEM) (Hitachi S-4800, Japan) and element analyzer (Thermo Scientific Flash EA 1112, America) were used. The element content of S was analyzed by barium salt titration method.
2.2 Synthesis of adsorbents
Adjacent hydroxyls can be oxidized into one pair of dialdehydes selectively by periodate[16], a typical preparation procedure has been proposed as follows. Take corn stalk as an example, firstly,0.02 kg of raw corn stalk powder (abbreviated as RCS) was added into 0.3 L NaOH solution (4%, w/v) and was activated for 24 h at 317 K under stirring. The synthesized products were washed with water thoroughly. The as-synthesized products, after vacuum drying, were marked as NaOH–S. Secondly, put the activated corn stalk into 0.2 L NaIO4 solution (10%, w/v) and adjusted the pH to approximate 3 with dilute sulfuric acid solution. Then the mixtures reacted in darkness for 24 h at 323 K under stirring for partial oxidation to introduce the dialdehydes. The synthesized products were washed with water thoroughly. After vacuum drying, the as-synthesized products are abbreviated as DA-S. Then 0.2 L of 10% aminothiourea (abbreviated as AT) was added into the as-synthesized DA-S reacted for 24 h at 343 K under stirring. The synthesized products were washed with ethanol and water thoroughly and then dried in vacuum, which were named as AT-S. Similarly, AT-P and AT-C derived from filter paper and cotton, respectively, were prepared by the same procedures as AT-S.
2.3 Adsorption
Au adsorption. A certain amount of Au(III) chloride solution with target concentration and acidity and dry adsorbents were added into a stoppered flask and reacted in a thermostatic oscillator (200 r/min). When the reaction stopped, solid and liquid phases were separated by centrifuge. After adsorption reached equilibrium, the metal ions concentration and pH were measured. Each group of the experiment was repeated twice, the average concentrations of metal ions were employed with the relative standard deviation (σ, %) less than 3% and the metal ions concentrations in the aqueous phase were repeatedly measured three times by ICP-OES with a relative standard deviation less than 3%. The Au-deposited AT-S was dried to carry out a series of characterizations such as XRD, TG, FTIR and SEM. Adsorption amounts (qe, mol/kg) were calculated as follows:
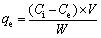 (1)
(1)
Adsorption selectivity: equal mole of Au(III) and Fe(III), Cu(II), Co(II), Ni(II), Pd(II), Pt(IV) were dissolved in a mixture solution with target concentration and acidity. And the adsorption procedure was the same as the Au(III) adsorption experiment. The relative separation coefficients between two kind of metal ions (R1/2) could be calculated as follows:
 (2)
(2)
 (3)
(3)
where Ce (mol/L) is the concentration of metal ion at equilibrium, Ci (mol/L) is the concentration of metal ion at initial time, W (kg) is the mass of the adsorbent, V (L) is the volume of liquid phase and Kd (kg/L) is the distribution coefficient.
3 Results and discussion
3.1 Characterization
Taking corn stalk as instance, the synthesis process has been characterized in detail. Figure 3 shows the infrared spectrograms of AT (aminothiourea), RCS, NaOH-S, DA-S, and AT-S.

Figure 3 Infrared spectra of AT, RCS, NaOH-S, DA-S, and AT-S
Table 1 describes their main infrared adsorption peaks and their corresponding groups.
Table 1 Assignment of main vibrational modes for adsorbents
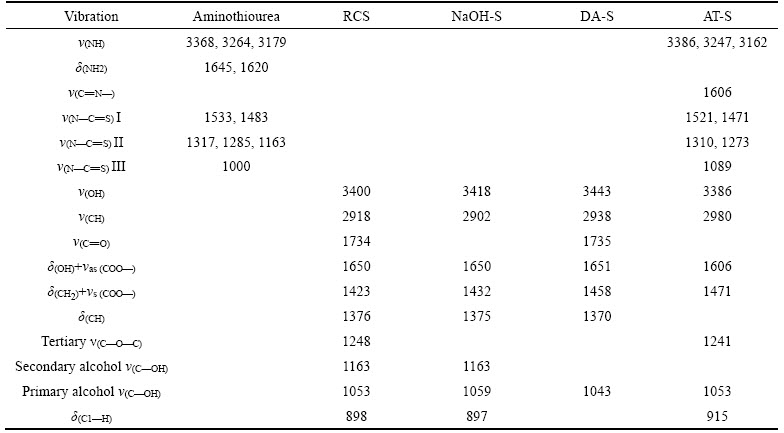
The characteristic peak of ν(COOH)(about 1734 cm–1) in the spectra of RCS illustrates that there are free —COOH groups in RCS. However, in the spectra of NaOH—S, the characteristic peak of ν(COOH) disappears but the two new peaks of ν(COO–) (about 1650 and 1432 cm–1) appear, proving that the free —COOH has been transformed into the ionic —COO– in NaOH—S. This can be concluded that the corn stalk powder has been activated by NaOH solution [17]. Compared to the spectra of NaOH—S, the peak strength of secondary alcohols derived from C2 and C3(about 1163 cm–1) weakens obviously, while two new peaks of ν(C=O) (about 1735 cm–1) and ν(C—H) (about 2938 cm–1) which belong to —CHO are observed in the spectra of DA-S, indicating that most adjacent —OH linking to C2 and C3 has been partially oxidized into the dialdehydes in DA-S [16]. Moreover, in the spectra of AT-S, the adsorption peak around 940–1140 cm–1, 1100–1420 cm–1 and 1395–1570 cm–1 can be assigned to characteristic vibration peaks of aminothiourea N—C=S, at the same time the —CHO characteristic peak (ν(C=O), 1735 cm–1) disappears. It proves that aminothiourea group has been grafted onto the structure of the corn stalk through the Schiff base reaction between —CHO and —NH2 [18].
Figure 4 shows the XRD spectra of RCS, NaOH—S, DA-S and AT-S. As seen, it shows that RCS and NaOH—S are in crystalline state. This may be explained by that there still exist the internal hydrogen bonds in NaOH—S and the crystal structure of corn stalk cannot be destroyed by NaOH treatment. Nevertheless, DA-S and AT-S are amorphous. It should attribute to that corn stalk can be oxidized by NaIO4 and the most hydrogen bonds in DA-S and AT-S are destructed. And the destruction of the hydrogen bonds in corn stalk is favorable for the adsorption of metal ions [17].

Figure 4 XRD patterns for RCS, NaOH-S, DA-S and AT-S
In addition, Table 2 depicts the adsorption capacity of Au(III) on every raw materials and derivatives of corn stalk, cotton and paper. Take corn stalk for example, the adsorption capacity of Au(III) gets improved consequently on RCS (0.056 mol/kg), NaOH-S (0.41 mol/kg), DA-S (0.70 mol/kg) and AT-S (20.0 mol/kg), respectively. However, the adsorption capacity is not too high for DA-S although there exist lots of oxygen- containing functional groups, such as —OH and —CHO. Probably, a weak interaction between oxygen-containing groups and AuCl4– in 1 mol/L HCl system leads to a lower adsorption capacity. Interestedly, the adsorption capacity of Au(III) on AT-S is around 357 times higher than that of RCS. This significant improvement demonstrates that aminothiourea grafted onto corn stalk is greatly favorable for adsorbing Au(III). Similarly, the adsorption capacities of Au(III) on their derivatives also get increased with different levels compared with raw materials. The capacities follow an increasing order as: NaOH— < dialdehyde— < aminothiourea—. However, AT-S shows the best adsorption properties for Au(III) among AT-S, AT-C and AT-P. This could be related with the content of aminothiourea groups in their structures. The elemental contents of C, H and N, and S of AT-S, AT-C and AT-P were characterized and listed in Table 3 in the SM, it can be found that the elemental mole ratios of nitrogen/sulfur for them are close to 1:3, which is consistent with the structure of aminothiourea unit. Therefore, it can roughly estimate the contents of aminothiourea groups in these final products according to the content of sulfur. Obviously, the content of aminothiourea groups in AT-S is higher than AT-C and AT-P. It displays that the more aminothiourea groups grafted onto adsorbents, the better the adsorption ability towards Au(III).
Table 2 Adsorption amounts (mol/kg) of related products for corn stalk, cotton and paper (Ci=0–27×10–3 mol/L, W=0.0110–3 kg, T=298.15 K, V=0.01 L, CHCl=1 mol/L, t=48 h)
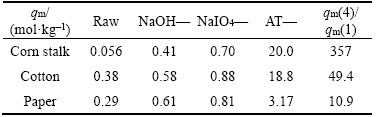
Table 3 Elemental contents of C, H, N and S for AT-S, AT-C and AT-P

3.2 Adsorption isotherm
The maximum adsorption capacities toward Au(III) by AT-S, AT-C and AT-P can be calculated according to the adsorption isotherms, as shown in Figure 5. In addition, the experimental data are fitted by Langmuir and Freundlich isotherm equations to estimate the maximum adsorption capacities of AT-S, AT-C and AT-P towards Au(III).
Langmuir isotherm equation [19]:
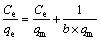 (4)
(4)
Freundlich isotherm equation [20]:
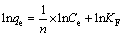 (5)
(5)
where b (L/mol) is the affinity constant, qm (mol/kg) is the maximum adsorption capacity, 1/n and KF (mol/kg) are the Freundlich constant relating to adsorption intensity and capacity, respectively.
The corresponding parameters are listed in Table 4. It can be seen that qm calculated by the Langmuir equation (qm,cal) is 21.4, 19.0 and 3.28 mol/kg for AT-S, AT-C and AT-P, respectively. And qm from the experimental (qm,exp) is 20.0, 18.8 and 3.17 mol/kg for AT-S, AT-C and AT-P, respectively. All the corresponding qm,cal and qm,exp are consistent with each other. Furthermore, all the correlation coefficients (R2) from the fitted Langmuir equation are also more than 0.99. It means that Langmuir isotherm equation can depict the experimental data rightly.
Figure 6 shows the relationship between the adsorption of Au(III) on AT-S, AT-C and AT-P and the adsorption temperature. As seen, it shows that the adsorption amount of Au(III) on the AT-S, AT-C and AT-P increases with the growing temperature. For example, the adsorption amount of Au(III) improves from 21.4 mol/kg at 298 K to 26.9 mol/kg at 328 K. The adsorption amount of Au(III) by AT-S, AT-C and AT-P and some other adsorbents reported elsewhere are listed in Table 5 [21–24]. Among these adsorbents, AT-S has the best adsorption ability towards Au(III) even at room temperature. It indicates that AT-S is a kind of promising adsorbent for Au(III).
3.3 Adsorption kinetics
Adsorption kinetics decides the adsorption efficiency and the minimum residence time. The adsorption kinetics of Au(III) on AT-S, AT-C and AT-P was thus investigated to estimate the minimum time to reach equilibrium. As shown in Figure 7, the adsorption kinetics of Au(III) are all quite fast at first and then approach equilibrium within 6 h. Generally, the adsorption kinetics can be analyzed by the following pseudo-first-order and pseudo-second-order models.
Pseudo first-order rate models [25]:
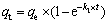 (6)
(6)
Pseudo second-order rate models [26]:
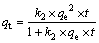 (7)
(7)
where qt (mol/kg) is the adsorption amount of Au(III) at time t, k1 (s-1) and k2 (kg·min/mol) are the first-order rate and second-order rate constant at equilibrium, respectively, t (min) is the adsorption time.
Their parameters for kinetic equations are calculated and summarized in Table 6, all fitting coefficients (R2) by the second-order rate equations are over 0.98 and higher than that by the first-order rate equations. Furthermore, for AT-S, AT-C and AT-P, the adsorption amounts of Au(III) at equilibrium evaluated from second-order equation (qe, cal) and the experimental data (qe, exp) are closer. It illustrates that the adsorption of Au(III) on AT-S, AT-C and AT-P can be depicted by pseudo- second-order kinetics model well. According to the assumption of this model, the rate-determining step should belong to the surface chemical reaction in this adsorption system.
Table 4 Fitting parameters of isotherm equations

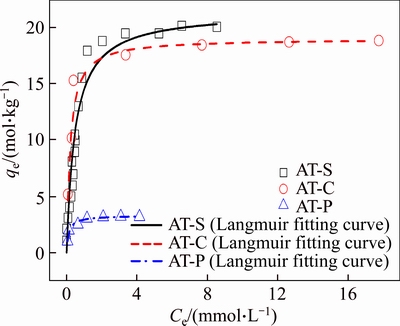
Figure 5 Adsorption isotherms and Langmuir fitting curves of Au(III) by AT-S, AT-C and AT-P (T=298.15 K, V=0.01 L, W=0.01×10–3 kg, CHCl=1 mol/L, t=48 h)
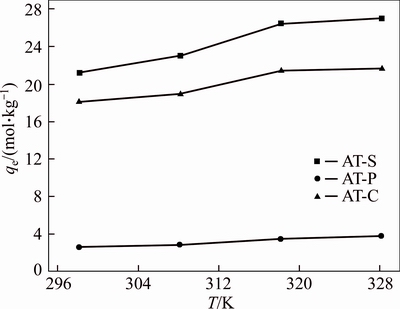
Figure 6 Effect of temperature on adsorption amount of Au(III) (W=0.01×10–3 kg, CAu=27.4, 21.9, 5.47×10–3 mol/L for AT-S, AT-C and AT-P, respectively. V= 0.01 L, CHCl=1 mol/L, t=48 h)
Table 5 Comparison of adsorption amount (q, mol/kg) of Au(III) on various adsorbents

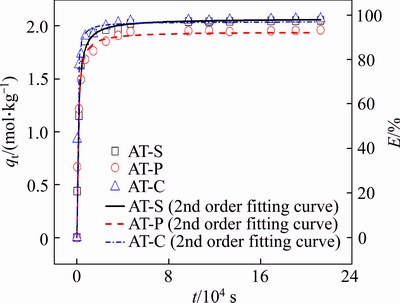
Figure 7 Adsorption kinetics and pseudo second-order kinetics fitting curves of Au(III) with AT-S, AT-C and AT-P (CAu=2×10–3 mol/L, W=0.05 kg, V=0.05 L, T=298.15 K, CHCl=1 mol/L)
Table 6 Fitting kinetic parameters for AT-S, AT-C and AT-P

3.4 Adsorption mechanism
Taking AT-S as an example, the adsorption mechanism was further elaborated. Figure 8 depicts the relationship between the adsorption of Au(III) on AT-S and the acidity of solution, which is helpful for elucidating the adsorption mechanism. The results show that the adsorption slightly declines when pH is between 2.0 and 4.0, and greatly descends since pH is higher than 4.0. In particular, AT-S exhibits an excellent adsorption behavior towards Au(III) when pH is lower than 2.0. Furthermore, the Zeta potential of AT-S particles varying with increasing pH is shown in Figure 9. It can be found that Zeta potential of AT-S decreases with the growing pH, and the isoelectric point is about 4.30 (pHPZC). This illustrates that the surface of AT-S particles displays different charge properties before and after pH 4.30, i.e., positive when pH<4.30 and negative when pH>4.30, indicating that there are various functional groups on the surface of AT-S particles.

Figure 8 Adsorption efficiencies of Au(III) at various pH(CAu=2×10–3 mol/L, W=0.01×10–3kg, T=298.15 K, V=0.01 L, t=48 h)
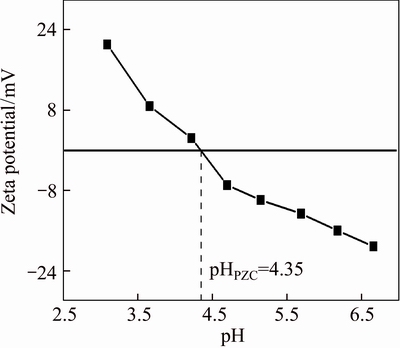
Figure 9 Zeta potential of the AT-S particles
Normally, there are several species for Au(III) calculated by the given cumulative stability constants (Formula (8)–(14)) in the mediates which contain Cl–. The predominance diagram of Au(III)- OH–-Cl– species are shown in Figure 10. From the figure, it can be seen that Au(III) mainly exists in the form of tridentate coordination complexes (AuClx(OH)3–x–) only when the concentration of Au(III) is very low (<0.1×10–3 mol/L), other than Au(III) exists in the form of tetra-coordination complex (AuClx(OH)4–x–). When the concentration of Cl– is more than 0.01 mol/L and the concentration of Au(III) is above 0.1×10–3 mol/L, AuCl4– is the main species of Au(III). With the growing pH of the solution, Au(III) will turn from AuCl4– to AuCl3(OH)– and AuCl2(OH)2–. In the UV-Vis characterization (Figure 10) of Au(III) in HCl mediate, blue-shifts of the LMCT (Ligand- Metal Charge-Transfer, at ~314 nm and ~220 nm) optical absorption peaks belonging to Au-Cl are observed, and the strengths of the peaks also turn weak at the same time. It proves that Au-Cl is replaced by Au-OH with the increasing pH. Generally, in various chloride leaching system (e.g., NaClO3+HCl, HCl+Cl2, aqua regia, and HCl+H2O2), the concentration of Au and HCl is higher than 0.1×10–3 and 0.01 mol/L, respectively. Hence, AuCl4– should be the main species of Au(III) in these leaching solution.
AuCl4–+OH–→AuCl3(OH)–+Cl–
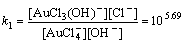 (8)
(8)
AuCl3+OH–→AuCl2(OH)+Cl–
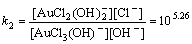 (9)
(9)
AuCl2(OH)2–+OH–→AuCl(OH)3–+Cl–
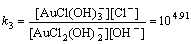 (10)
(10)
AuCl2(OH)2–+OH–→AuCl(OH)3–+Cl–
 (11)
(11)
AuCl3(OH)–+OH–→AuCl2(OH)2–+Cl–
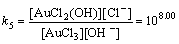 (12)
(12)
AuCl2(OH)+OH–→AuCl(OH)2+Cl–
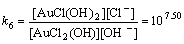 (13)
(13)
AuCl(OH)2+OH–→Au(OH)3+Cl–
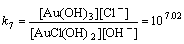 (14)
(14)
According to the species distribution of Au(III) in HCl solutions, Au(III) mainly exists in the form of AuClxOH(4–x)– or AuCl4– when the concentration of Au(III) is higher than 10–4 mol/L. Hence, when pH is lower than 4.0, the positive-charged active sites of AT-S are beneficial to capture the negative- charged gold complex anion AuClxOH(4–x)– or AuCl4–, thus, displaying a high adsorption efficiency. When pH>4.0, there is an electrostatic repulsive action between the negative-charged active sites of AT-S and the anion species AuClxOH(4–x)– or AuCl4–. Hence, the adsorption efficiency declines at a higher pH of more than 4.0. Moreover, the increasing electrostatic repulsive force results in the declining adsorption with the further growing pH. However, besides the electrostatic attraction, the coordination between thiourea groups and Au(III) is another important factor (lgAu-thiourea k2=21.95 [28]). Hence, the adsorption efficiency can still maintain around 80% even there exists an electrostatic repulsive force when pH>4.0.
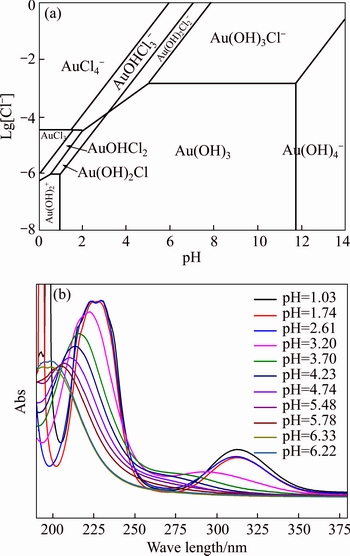
Figure 10 Predominance diagram of Au(III)-OH–-Cl– species [27] (a) and UV-VIS spectra of 2×10–3 mol/L HAuCl4 at various pH (b)
In addition, X-ray diffraction analysis (XRD) was used to confirm the status of the adsorbed Au on the AT-S. Thus, XRD patterns of AT-S before and after adsorption of Au(III) are shown in Figure 11. For AT-S, there is no obvious diffraction peak of crystals, which means that AT-S is amorphous. However, for AT-S after adsorption of Au(III) (AT-S-Au), some sharp peaks (around 38.18°, 44.39°, 64.57° and 77.54°) belonging to the crystal structure of metallic gold appears, which verifies that metallic gold has generated and deposited on the AT-S. The curves of thermogravimetry (TG) as well as derivative thermogravimetry (DTG) of AT-S-Au in air (310– 1273 K) are plotted in Figure 12. DTG curve displays three mass-loss peaks at 500.85, 577.95 and 877.15 K, respectively, which probably corresponds to the decomposition of amino-thiourea groups, the oxygenated groups and the combustion of the skeleton carbon of AT-S, respectively. The residue mass fraction of gold is around 59.4% from TG curve at 877.15 K. Furthermore, the SEM images of AT-S and AT-S-Au are shown in Figure 13. It confirms that the AuCl4– adsorbed by AT-S can be reduced to elemental gold particles and deposit on the surface of the AT-S.
It has been reported that the AuCl4– is reduced to elemental gold and meantime the hydroxyl groups on biosorbents are simultaneously oxidized to be carbonyl groups, such as algal [29], saccharides [5], cotton cellulose [12], lignin [23], tannin [30], and hydrothermal carbon spherules [31]. In this work, the reduction groups of R–OH on the AT-S might also took part in the Au(III) reduction process:
 —OH→Au(0)+4Cl–+3R(O)+3H+
—OH→Au(0)+4Cl–+3R(O)+3H+
Therefore, according to the above discussion, AT-S firstly complex with AuCl4– through two routes. One is electrostatic attraction between the protonated R-NH3+ and AuCl4–, and the other is coordination between amino thiourea groups and AuCl4–. Then, the adjacent hydroxyl groups on the AT-S can reduce the adsorbed AuCl4– to be Au(0), finally the produced elemental gold particles deposit on the AT-S. Here, the reduction–deposition coupled mechanism can be described in Figure 14.

Figure 11 XRD patterns of AT-S and AT-S-Au
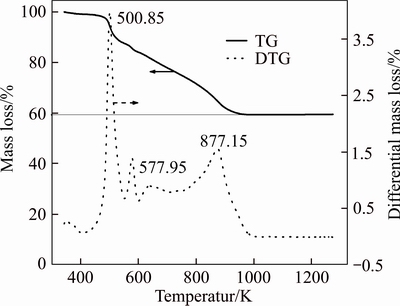
Figure 12 TG-DTG curves for AT-S-Au

Figure 13 SEM images of AT-S(a)and AT-S-Au (b)
3.5 Adsorption selectivity
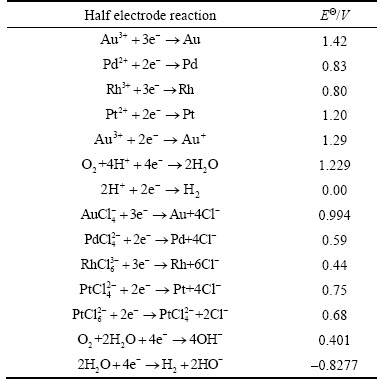
The separation coefficients (R) between Au(III) and Pt(VI), Pd(II), Rh(III), Cu(II), Ni(II) or Co(II) by AT-S under different acidities are listed in Table 7. It is well known that Ni(II), Cu(II), Co(II) and Rh(III) mainly exist in the forms of positive-charged species, while AT-S also presents a positive-charged form due to the protonation under the higher acidity. In addition, the weak coordination ability between thiourea groups and Co(II), Ni(II), or Rh(III) also leads to lower adsorption. However, the coordination between Cu(II) and thiourea groups are much stronger (lgCu-thiourea k4=16.4 [28]). Thus, the separation coefficients between Au(III) and Co(II), Ni(II) or Rh(III) are prominent rather than Cu(II). On the other hand, for Pt(VI) and Pd(II) in chloride system, their major species are anion complexes PdClxOH(4–x)2– and PtClxOH(6–x)2–, respectively. In addition, it has been reported that thiourea groups or their derivatives can also coordinate with Pd(II) and Pt(VI) [32]. Furthermore, as shown in Table 8, the standard electrode potentials of Au(III), Pt(IV) and Pd(II) is very high. Similar to Au(III), it is easy for Pt(IV) and Pd(II) to be reduced during the process of adsorption. Therefore, the selectivity between Au(III) and Pd(II) or Pt(VI) are not good enough, particularly for Pt(VI). However, AT-S can adsorb Au(III) with a satisfying selectivity under the higher acidity (e.g., at pH 0.00).
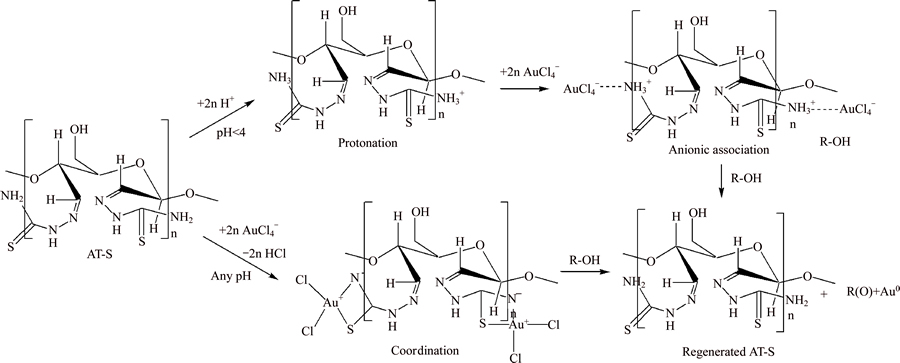
Figure 14 Deduced reduction–deposition coupled mechanism for Au(III) absorption
Table 7 Separation factors between Au(III) and other related metal ions (CM=0.1×10–3 mol/L, W=0.01× 10–3 kg, V=0.01 L, T=298.15 K, t=48 h)

Table 8 Standard electrode potential for complexes of precious metal ions with Cl– ligands [28]
4 Conclusions
In this work, a general strategy is proposed firstly for waste cellulosic biomass, to be modified with aminothiourea groups through periodate partial oxidation followed by schiff reaction. It has been found that aminothiourea-modified corn stalks (AT-S) show a superior adsorption performance towards Au(III). To our knowledge, AT-S has been reported the highest adsorption capacity towards Au(III) even at room temperature. Furthermore, an reduction-deposition mechanism has been proposed. AT-S can complex with AuCl4– through electrostatic attraction between the protonated R-NH3+ and AuCl4– or coordination between amino thiourea groups and AuCl4–, where the coordination by amino thiourea groups plays a main role for the adsorption. Then, the hydroxyl groups on the AT-S reduce the adsorbed AuCl4– to be Au(0), finally the formed elemental gold particles deposit on the AT-S. In addition, AT-S shows a superior selectivity towards Au(III) under the higher acidity. The current work shows a good prospect for AT-S to be an valid biosorbent for recovery of gold and the possible fabrication of gold nanoparticles by reduction-deposition process without any reductant.
References
[1] DAS N. Recovery of precious metals through biosorption — A review [J]. Hydrometallurgy, 2010, 103(1–4): 180–189.
[2] LI X B, YE J J, LIU Z H, QIU Y Q, LI L J, MAO S, WANG X C, ZHANG Q. Microwave digestion and alkali fusion assisted hydrothermal synthesis of zeolite from coal fly ash for enhanced adsorption of Cd(II) in aqueous solution [J]. Journal of Central South University, 2018, 25(1): 9–20.
[3] TIAN Q H, WANG X Y, MAO F F, GUO X Y. Absorption performance of DMSA modified Fe3O4@SiO2 Core/Shell magnetic nanocomposite for Pb2+ Removal [J]. Journal of Central South University, 2018; 25(4): 709–718.
[4] LI J H, MIAO X X, CHEN X Y, LU L, YANG Y, FU Y Q, XIONG C H. Application and characterization of grafted polytetrafluoroethylene fiber for enhanced adsorption of Cu(II) in aqueous solutions [J]. Journal of Central South University, 2016, 23(10): 2513–2519.
[5] PANGENI B, PAUDYAL H, ABE M, INOUE K, KAWAKITA H, OHTO K, ADHIKARI B B, ALAM S. Selective recovery of gold using some cross linked polysaccharide gels [J]. Green Chem, 2012, 14(7): 1917– 1927.
[6] GURUNG M, ADHIKARI B B, GAO X, ALAM S, INOUE K. Sustainability in the metallurgical industry: Chemically modified cellulose for selective biosorption of gold from mixtures of base metals in chloride media [J]. Ind Eng Chem Res, 2014, 53(20): 8565–8576.
[7] PARAJULI D, ADHIKARI C R, KAWAKITA H, KAJIYAMA K, OHTO K, INOUE K. Reduction and accumulation of Au(III) by grape waste: A kinetic approach [J]. React Funct Polym, 2008, 68(8): 1194–1199.
[8] XIONG Y, ADHIKARI C R, KAWAKITA H, OHTO K, INOUE K, HARADA H. Selective recovery of precious metals by persimmon waste chemically modified with dimethylamine [J]. Bioresour Technol, 2009, 100(18): 4083–4089.
[9] FAN R, FENG X, GUAN X, ZHANG Q, LUO Z. Selective adsorption and recovery of Au(III) from three kinds of acidic systems by persimmon residual based bio-sorbent: A method for gold recycling from E-Wastes [J]. Bioresour Technol, 2014, 163(7): 161–171.
[10] PING Y, XU M, QU R, HOU C, LIU X, JIANG Z, QIANG X. Uptake of Gold (III) from waste gold solution onto biomass-based adsorbents organophosphonic acid functionalized spent buckwheat hulls [J]. Bioresour Technol, 2013, 128(1): 36–43.
[11] PANGENI B, PAUDYAL H, INOUE K, KAWAKITA H, OHTO K, ALAM S. An Assessment of gold recovery processes using cross-linked paper gel [J]. Journal of Chemical and Engineering Data, 2012, 57(3): 381–391.
[12] PANGENI B, PAUDYAL H, INOUE K, KAWAKITA H, OHTO K, ALAM S. Selective recovery of Gold(III) using cotton cellulose treated with concentrated sulfuric acid [J]. Cellulose, 2012, 19(2): 381–391.
[13] ZUO G, ORECCHIO S, MUHAMMED M. Facilitated transport of gold through a membrane via complexation to thiourea-based reagents [J]. Sep Sci Technol, 1996, 31(11): 1597–1613.
[14] ZUO G, MUHAMMED M. Thiourea-based coordinating polymers: Synthesis and binding to noble metals [J]. React Polym, 1995, 24(3): 165–181.
[15] YIRIKOGLU H, GULFEN M. Separation and recovery of Silver(I) ions from base metal ions by melamine-formaldehyde-thiourea (MFT) chelating resin [J]. Sep Sci Technol, 2008, 43(2): 376–388.
[16] JACKSON E L, HUDSON C S. Application of the cleavage type of oxidation by periodic acid to starch and cellulose [J]. J Am Chem Soc, 1937, 59(10): 2049–2050.
[17] ZHENG L, DANG Z, YI X, ZHANG H. Equilibrium and kinetic studies of adsorption of Cd(II) from aqueous solution using modified corn stalk [J]. J Hazard Mater, 2010, 176(1–3): 650–656.
[18] CORDES E H, JENCKS W P. On the mechanism of schiff base formation and hydrolysis [J]. J Am Chem Soc, 1962, 84(5): 832–837.
[19] LANGMUIR I. The constitution and fundamental properties of solids and liquids.
[20] FREUNDLICH H M F. Ueber die adsorption in lesungen [J]. Z Phys Chem, 1906, 57A: 385–470. (in Germany)
[21] HE Z W, HE L H, YANG J, LU Q F. Removal and recovery of Au(III) from aqueous solution using a low-cost lignin-based biosorbent [J]. Ind Eng Chem Res, 2013, 52(11): 4103–4108.
[22] GURUNG M, ADHIKARI B B, KAWAKITA H, OHTO K, INOUE K, ALAM S. Recovery of Au(III) by using low cost adsorbent prepared from persimmon tannin extract [J]. Chem Eng J, 2011, 174(2, 3): 556–563.
[23] PARAJULI D, KHUNATHAI K, ADHIKARI C R, INOUE K, OHTO K, KAWAKITA H, FUNAOKA M, HIROTA K. Total recovery of gold, palladium, and platinum using lignophenol derivative [J]. Miner Eng, 2009, 22(13): 1173–1178.
[24] DONIA A M, ATIA A A, ELWAKEEL K Z. Recovery of Gold(III) and silver(I) on a chemically modified chitosan with magnetic properties [J]. Hydrometallurgy, 2007, 87(3, 4): 197–206.
[25] LAGERGREN S. Zur theorie der sogenannten adsorption gel ster stoffe [J]. K Sven Vetenskapsakad Handl, 1898, 24(4): 1–39. (in Swedish)
ster stoffe [J]. K Sven Vetenskapsakad Handl, 1898, 24(4): 1–39. (in Swedish)
[26] HO Y S. Adsorption of Heavy Metals from Waste Streams by Peat [D]. Birmingham, UK: University of Birmingham, 1995.
[27] YU J, CHENG R. Precious metal extraction chemistry [M]. Beijing, China: Beijing Chemical Industry Press, 2010. (in Chinese)
[28] SPEIGHT J G. Lange’s handbook of chemistry [M]. 16th Edition, New York: McGraw-Hil, 1998.
[29] MATA Y N, TORRES E, BL ZQUEZ M L, BALLESTER A, GONZ
ZQUEZ M L, BALLESTER A, GONZ LEZ F, MU
LEZ F, MU OZ J A. Gold(III) biosorption and bioreduction with the brown alga fucus vesiculosus [J]. J Hazard Mater, 2009, 166(2, 3): 612–618.
OZ J A. Gold(III) biosorption and bioreduction with the brown alga fucus vesiculosus [J]. J Hazard Mater, 2009, 166(2, 3): 612–618.
[30] OGATA T, NAKANO Y. Mechanisms of gold recovery from aqueous solutions using a novel tannin gel adsorbent synthesized from natural condensed tannin [J]. Water Res, 2005, 39(18): 4281–4286.
[31] WANG F, ZHAO J, ZHU M, YU J, HU Y S, LIU H. Selective adsorption-deposition of gold nanoparticles onto monodispersed hydrothermal carbon spherules: A reduction-deposition coupled mechanism [J]. J Mater Chem A, 2015, 3(4): 1666–1674.
[32] WANG S. Synthesis of Polymeric Ester Thiourea Resin and Its Adsorption and Separation Properties for Noble Metal Ions [D]. Changsha, China: Central South University, 2008. (in Chinese)
(Edited by FANG Jing-hua)
中文导读
氨基硫脲功能化PGMA及对金的吸附性能
摘要:纤维素基废弃生物质具有价格低廉和环境友好等优点,因而得到了广泛应用。本文提出了一种纤维素基废弃生物质功能化的通用制备方法,即先通过NaIO4选择性氧化将废弃生物质转化为含双醛基的平台中间体,然后利用醛基特定的Schiff base反应引入对金离子有优良配位能力的配基。以典型的废弃纤维素基生物质,如玉米秸秆 (AT-S)、棉花 (AT-C) 和纸 (AT-P) 为原料制备了氨基硫脲修饰的吸附剂。AT-S、AT-C和AT-P在298 K下对Au(III) 的饱和吸附容量分别为21.4、19.0和3.28 mol/kg,Au(III)吸附量与吸附剂中硫脲官能团的含量成正相关性。Au(III)的吸附符合准二级动力学吸附模型和Langmuir等温吸附模型。AT-S对Au(III)的吸附量是玉米秸秆的357倍,是目前文献中报道的有关Au(III) 吸附容量最高的吸附剂。AT-S对Au(III)与其他金属如Cu(II)、Ni(II)、Co(II)、Pt(VI)、Pd(II)和Rh(III)有着优良的分离选择性。采用XRD、TG、SEM、TEM和FTIR进行分析表征,结果表明吸附的AuCl4– 被还原为Au0纳米颗粒并沉积在AT-S吸附剂表面。
关键词:吸附;还原-沉积;废弃纤维素基生物质;氨基硫脲;金纳米颗粒
Foundation item: Projects(51504073, 51404081, 51672275) supported by the National Natural Science Foundation of China; Project(2012CBA01202) supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology, China; Project (QianJiaoKeHe KY[2015]433) supported by the Research Program of the Education Department of Guizhou Province, China; Project(XJG20141104) supported by the Research Program of Talented Scholars of Guizhou Institute of Technology, China
Received date: 2017-10-10; Accepted date: 2018-05-04
Corresponding author: ZHAO Jun-mei, PhD, Associate Professor; Tel: +86-10-82544911; E-mail: jmzhao@ipe.ac.cn; ORCID: 0000-0001-6809-5032

