氨性体系超声耦合锌电加强置换提镉工艺及阳极反应机理
来源期刊:中国有色金属学报(英文版)2019年第9期
论文作者:南天翔 杨建广 汪文超 李陵晨 杨建英
文章页码:1967 - 1974
关键词:镉;电加强置换;超声波;电化学机理;漂浮海绵镉
Key words:cadmium; electrically enhanced cementation; ultrasonic; electrochemical mechanism; floating sponge cadmium
摘 要:采用单因素实验法研究有超声耦合时氨性体系中锌电加强置换提镉的优化工艺条件。结果表明,在温度35 °C、阴阳两极极板面积比1:2、阳极电流密度15 A/m2、耦合超声频率为40 kHz、超声功率100 W条件下以锌板为阳极置换溶液中的镉6 h,镉的置换率为99.21%,且显著抑制“漂浮海绵镉”的产生。采用循环伏安法和线性扫描伏安法,对比研究氨性体系下有/无超声耦合时锌电加强置换提镉的阳极反应机理。循环伏安法表明超声波能促进置换反应的进行,加快置换反应速率,减小电加强置换反应所需电压,改变反应控制步骤;线性扫描伏安法表明,温度及超声功率的增加均能够促进电加强置换反应的进行,加快反应速率,降低置换自发反应所需电位。
Abstract: Cadmium was replaced by zinc in ammoniacal system using an electrically enhanced method under ultrasonic waves. Five main influencing factors were investigated by a single-factor experiment to determine the optimum parameters. Cyclic voltammetry and linear sweep voltammetry were applied to investigating the reaction mechanism of electrically enhanced cementation of cadmium on a zinc plate. The optimum parameters were a temperature of 35 °C, a cathode-to-anode area ratio of 1:2, an anode current density of 15 A/m2, an ultrasonic frequency of 40 kHz a reaction time of 6 h and an ultrasonic power of 100 W. The extraction rate was 99.21%, and the production of byproduct “floating sponge cadmium” was inhibited. The analysis of the cyclic voltammetry and linear sweep voltammetry diagrams showed that ultrasonic waves can promote and accelerate the replacement reaction, decrease the voltage requirement of the electrically enhanced replacement reaction, and change the reaction steps. In addition, increasing the temperature and ultrasonic power can promote and accelerate electrically enhanced replacement reactions and decrease the electric potential requirement.
Trans. Nonferrous Met. Soc. China 29(2019) 1967-1974
Tian-xiang NAN1, Jian-guang YANG1, Wen-chao WANG1, Ling-chen LI 1, Jian-ying YANG2
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Environmental Engineering Vocational College, Ganzhou 342000, China
Received 18 November 2018; accepted 19 April 2019
Abstract: Cadmium was replaced by zinc in ammoniacal system using an electrically enhanced method under ultrasonic waves. Five main influencing factors were investigated by a single-factor experiment to determine the optimum parameters. Cyclic voltammetry and linear sweep voltammetry were applied to investigating the reaction mechanism of electrically enhanced cementation of cadmium on a zinc plate. The optimum parameters were a temperature of 35 °C, a cathode-to-anode area ratio of 1:2, an anode current density of 15 A/m2, an ultrasonic frequency of 40 kHz a reaction time of 6 h and an ultrasonic power of 100 W. The extraction rate was 99.21%, and the production of byproduct “floating sponge cadmium” was inhibited. The analysis of the cyclic voltammetry and linear sweep voltammetry diagrams showed that ultrasonic waves can promote and accelerate the replacement reaction, decrease the voltage requirement of the electrically enhanced replacement reaction, and change the reaction steps. In addition, increasing the temperature and ultrasonic power can promote and accelerate electrically enhanced replacement reactions and decrease the electric potential requirement.
Key words: cadmium; electrically enhanced cementation; ultrasonic; electrochemical mechanism; floating sponge cadmium
1 Introduction
In nature, cadmium resources exist in zinc ores, lead-zinc ores and copper-lead-zinc ores. Most cadmium remains reserved in lead-zinc concentrates after flotation [1]. At present, some lead and zinc smelteries have a mass of raw materials with high cadmium content, leading to a great deal of cadmium remaining in the lead-zinc concentrates. An analysis of cadmium pollution sources shows that lead-zinc concentrate smelting accounts for 70% of the total cadmium pollution. The cadmium dust and smelting slag produced by lead-zinc smelting are major cadmium pollution sources [2]. The roasting of lead-zinc concentrates, leaching slag volatilization in rotary kilns, ISP system sintering and oxygen enrichment strengthening smelting of lead concentrates can produce large amounts of smoke dust containing cadmium, and in the smoke dust, the cadmium content varies from 2% to 25% [3]. Thus, it is of crucial significance to develop clean and efficient process procedures for emission reduction and pollution headstream control [4].
In China, most smelting enterprises mainly incorporate the treatment of smoke dust containing cadmium into the zinc acid smelting system using the technological route of “acid leaching-zinc replacement- floating sponge cadmium briquetting-cadmium refining” to carry out the cadmium removal process [5]. However, the bottleneck problem is that it is difficult to solve the problems of “cadmium-coated zinc” and “copper-coated zinc” when zinc displaces cadmium. A single replacement cycle results in sponge cadmium that is only 30% to 50%, which is far lower than the requirement of 80% for floating sponge cadmium briquetting [6]. Some new methods have been proposed, including zinc powder replacement [7], electrodeposition [8], adsorption [9] and solvent extraction [10]. The above methods have advantages, but there are still many limitations, such as long and inefficient technological routes, difficult separation of zinc and cadmium, high zinc powder consumption and low cadmium recovery. Additionally, serious environmental pollution risk still exists from dispersed cadmium lost to different waste liquids and various metallurgical slags during a process [11].
YANG et al [12] proposed a new technology, electrically enhanced replacement, for cadmium in ammoniacal system, and this process can realize clean and efficient extraction of cadmium in an ammoniacal solution containing cadmium. However, “floating sponge cadmium” was easily produced in the process of electrically enhanced replacement of cadmium in subsequent studies. The large amount of “floating sponge cadmium” can cover the anode plate and cathode plate and even cause short circuits of the electrode in the late stage of the electrically enhanced replacement reaction [13]. Moreover, the “floating sponge cadmium” can be easily oxidized to obtain compact cadmium clusters, which reduces the efficiency of cadmium extraction.
Ultrasonic waves are widely used in medical and industrial fields in recent years for their high energy [14]. Due to the special deconcentration polarization [15] and cavitation effect [16], extensive studies and ultrasonic practices have been applied in the fields of electroplating [17], metallurgy [18] and material preparation [19]. In this work, ultrasonic waves were applied to the proposed electrically enhanced replacement and cadmium extraction system, and the optimum technological conditions with or without ultrasonic waves were studied. First, the single factor test method was used to optimize the process conditions, and the effects of various factors on the efficiency of cadmium extraction were analyzed to determine the optimum technological conditions. Then, the anode electrochemical reaction mechanism for the extraction of cadmium through electrically enhanced replacement with or without ultrasonic coupling was studied. The comparison of electrochemical curves with or without ultrasonic coupling revealed the inhibition mechanism of “floating sponge cadmium” and provides a reference for peer research.
2 Experimental
2.1 Test reagents, solution preparation and test equipment
All experiments were performed in a three-electrode cell. All solutions were prepared using distilled water with analytically pure reagents. The solution contained 20 g/L Cd2+ (CdCl2) and 50 g/L NH4Cl in all tests.
2.2 Process experiment
The optimum technological conditions for the extraction of cadmium through electrically enhanced replacement of zinc in ammoniacal system were studied by using a single factor test method. Temperatures ranging from 25 to 40 °C, cathode-to-anode area ratios ranging from 1:2 to 1:4, anode current densities ranging from 15 to 25 A/m2, and ultrasonic powers ranging from 0 to 100 W were tested separately to study the optimum technological conditions for the replacement of cadmium.
2.3 Electrochemical experiments
The experiments were conducted at 298-328 K (25-45 °C) with an ultrasonic power in the range of 0-100 W and ultrasonic frequency of 40 kHz (ultrasonic cleaning bath provided by Kunshan Meimei Ultrasonic Instruments Company, China) in an oxygen-free electrolyte, which was purged with nitrogen to displace oxygen. All electrochemical measurements were performed using a computer-controlled potentiostat/ galvanostat (CHI670E electrochemical work station provided by Shanghai CH Instruments Company, China). A zinc electrode with a surface area of 19.625 mm2 was used as the working electrode. Before each measurement, the working electrode was polished using 1200-grit and 3500-grit sandpaper and then rinsed with absolute ethanol and distilled water. A graphite electrode with a surface area of 1.563 cm2 was used as the counter electrode, and a saturated calomel electrode (SCE) was used as the reference electrode for all potentials in this study (Fig. 1).
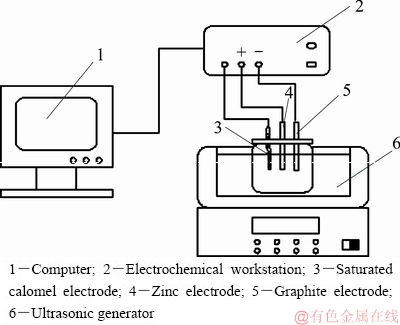
Fig. 1 Schematic diagram of electrochemical testing apparatus
3 Results and discussion
3.1 Process experiments
3.1.1 Effect of temperature on cadmium extraction
The cadmium extraction efficiency was tested at temperatures of 25, 30, 35 and 40 °C with a cathode-to- anode area ratio of 1:1, anode current density of 10 A/m2, reaction time of 3 h, NH4Cl concentration of 50 g/L, Cd2+ concentration of 20 g/L and ultrasonic power of 0 W. The test results are shown in Fig. 2.
The test results show that the replacement rate of cadmium remains stable with increasing temperature, peaking at 87.31%, when the temperature is 35 °C, and declines to 78.80% when the temperature is 40 °C because the formation of metal cadmium from cadmium ions by receiving an electron is an endothermic reaction. The reaction can more easily occur with a high temperature. However, a high concentration of H+ in the solution is produced by the accelerated hydrolysis of NH4+ in solution at high temperature, leading to a strong reaction between Zn and H+, causing a lower cadmium extraction efficiency. The other phenomenon observed during the tests was that more bubbles were released from the zinc anode at the higher temperatures. Based on a comprehensive consideration, the optimum temperature for cadmium extraction is 35 °C.
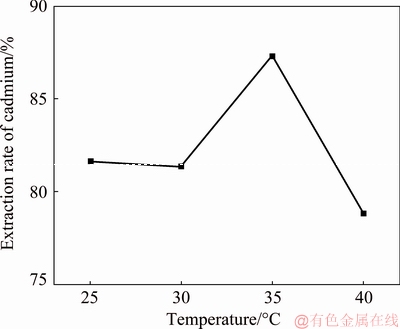
Fig. 2 Cadmium extraction efficiency at different temperatures
3.1.2 Effect of reaction time on cadmium extraction
The cadmium extraction efficiency was tested under different reaction time of 3, 4, 5, 6 and 7 h with a cathode-to-anode area ratio of 1:1, an anode current density of 10 A/m2, a temperature of 25 °C, a concentration of NH4Cl of 50 g/L, a concentration of Cd2+ of 20 g/L and an ultrasonic power of 0 W. The test results are shown in Fig. 3. It needs to be emphasized that the temperature has a certain influence on the cadmium extraction rate, as verified by Section 3.1.1. In the subsequent single factor experiments, the reaction temperature was set at 25 °C to highlight the effect of the reaction time on the efficiency of cadmium extraction. The test results are shown in Fig. 3.
The test results show that the replacement rate of cadmium gradually increases with increasing reaction time. The extraction yield of cadmium is 95.86% when the reaction time is 6 h. By continuing to increase the reaction time, the extraction yield of cadmium reaches 95.92% at 7 h. The extraction yield of cadmium reaches a maximum at 6 h and the extraction yield of cadmium was not significantly increased by prolonging the reaction time. Based on comprehensive considerations, the optimum reaction time for cadmium extraction is 6 h.
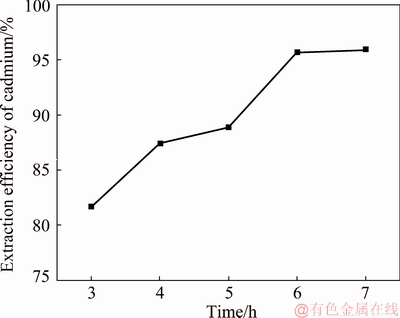
Fig. 3 Cadmium extraction efficiency at different reaction time
3.1.3 Effect of cathode-to-anode area ratio on cadmium extraction
The cadmium extraction efficiency was tested with different cathode-to-anode area ratios of 1:1, 1:2, 1:3 and 1:4 with a reaction time of 3 h, an anode current density of 10 A/m2, a temperature of 25 °C, a concentration of NH4Cl of 50 g/L, a concentration of Cd2+ of 20 g/L and an ultrasonic power of 0 W. It needs to be emphasized that the reaction time has a prominent influence on the cadmium extraction rate, as verified by Section 3.1.2. The extraction yield of cadmium is 95.86% when the reaction time is 6 h. In the subsequent single factor experiments, the reaction time was set to 3 h to highlight the effect of the cathode-to-anode area ratio on the efficiency of cadmium extraction. The test results are shown in Fig. 4.
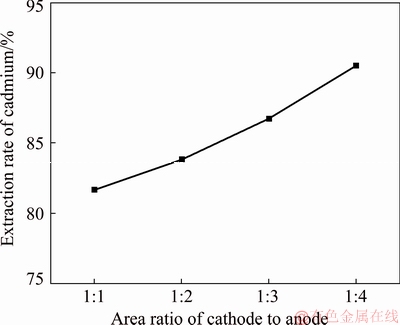
Fig. 4 Cadmium extraction efficiency at different cathode-to- anode area ratios
Figure 4 shows that the replacement rate of cadmium gradually increases with increasing cathode-to- anode area ratio. The extraction yield of cadmium is 90.52% when the cathode-to-anode area ratio is 1:4. The reasons for this result are as follows: the contact area of Zn in solution increases with the increasing cathode-to-anode area ratio. Under the same conditions, the ratio of replacement with Cd2+ in the solution is larger and the reaction rate is faster. However, during the reaction process, when the area of the anode plate increases, the possibility of zinc detachment from the zinc plate increases, resulting in a decrease in the purity of the sponge cadmium. At the same time, the increase in area causes the current and voltage to increase at the same current density, so the energy consumption increases as well, which is not conducive to cost savings. Based on comprehensive considerations, the optimum cathode-to- anode area ratio for cadmium extraction is 1:2.
3.1.4 Effect of cathode current density on cadmium extraction
The cadmium extraction efficiency was tested at different anode current densities of 5, 10, 15, 20 and 25 A/m2 with a reaction time of 3 h, a cathode-to-anode area ratio of 1:1, a temperature of 25 °C, a concentration of NH4Cl of 50 g/L, a concentration of Cd2+ of 20 g/L, and an ultrasonic power of 0 W. It needs to be emphasized that the cathode-to-anode area ratio has a prominent influence on the cadmium extraction rate, as verified by Section 3.1.3. In the subsequent single factor experiment, the cathode current density was set at 1:1 to highlight the effect of the cathode current density on the efficiency of cadmium extraction. The test results are shown in Fig. 5.
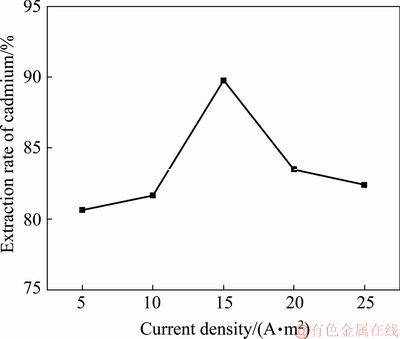
Fig. 5 Cadmium extraction efficiency at different anode current densities
Figure 5 shows that the extraction yield of cadmium first increases and then decreases with increasing cathode current density. The maximum cadmium extraction rate is 89.77% when the current density is 15 A/m2. The reasons for this result can be listed as follows: under the condition without current, a small amount of current will be produced from the zinc plate by the replacement reaction, and a small amount of current will enhance the replacement reaction. If the current is too high, the reaction will change from the replacement reaction with Cd to the dissolution of Zn, leading to a lower cadmium extraction efficiency. Based on comprehensive considerations, the optimum cathode current density for cadmium extraction is 15 A/m2.
3.1.5 Effect of ultrasonic power on cadmium extraction
The cadmium extraction efficiency was tested with different ultrasonic powers of 0, 20, 40, 60, 80 and 100 W with a reaction time of 3 h, a cathode-to-anode area ratio of 1:1, a temperature of 25 °C, a current density of 10 A/m2, a concentration of NH4Cl of 50 g/L, and a concentration of Cd2+ of 20 g/L. The test results are shown in Fig. 6.
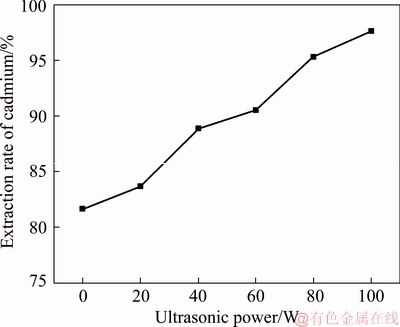
Fig. 6 Cadmium extraction efficiency at different ultrasonic powers
Figure 6 shows that the replacement rate of cadmium gradually increases with increasing ultrasonic power. The maximum cadmium extraction rate is 97.64% when the ultrasonic power is 100 W. Compared to the other factors, the ultrasonic power significantly increases the efficiency of cadmium extraction. Ultrasonic coupling can inhibit the production of “floating sponge cadmium”. The optimum ultrasonic power for cadmium extraction is 100 W.
3.1.6 Comparison experiments
The extraction of cadmium with a temperature of 25 °C, cathode-to-anode area ratio of 1:2, a current density of 15 A/m2, and a reaction time of 6 h was tested with or without ultrasonic coupling using the optimum technological conditions. The efficiency of cadmium extraction and the morphology of “floating sponge cadmium” under ultrasonic coupling conditions were also tested. Table 1 lists the efficiency of cadmium extraction. Figures 7 and 8 show the morphology of cadmium from electrically enhanced replacement with or without ultrasonic coupling.
Table 1 Technological parameters of cadmium extraction by microcurrent with and without ultrasonic coupling
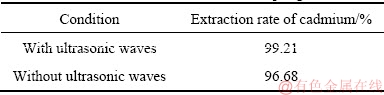

Fig. 7 Photograph of cadmium after replacement reaction with ultrasonic waves

Fig. 8 Photograph of cadmium after replacement reaction without ultrasonic waves
Comparing the cadmium extraction with or without ultrasonic coupling indicates that ultrasonic coupling can enhance and accelerate the replacement rate. A comparison of the morphology of the cadmium extracted by electrically enhanced replacement with or without ultrasonic coupling indicates that “floating sponge cadmium” appears without ultrasonic coupling but is not present with ultrasonic coupling. The reasons for this difference can be listed as follows: after the separation of cadmium from the anode without ultrasonic coupling, the anode surface is covered with a layer of cadmium, which leads to an increase in the bath voltage. Because of the increase in the bath voltage, the anode reaction changes to a gas evolution reaction, and the cadmium layers containing gas become “floating sponge cadmium”. However, ultrasonic waves are sonic waves with energy, which can cause cavitation in solution. The cadmium generated on the anode no longer covers the anode surface with ultrasonic coupling; in other words, there is no gas evolution reaction, which can reduce or even eliminate the formation of “floating sponge cadmium”.
3.2 Electrochemical experiments
3.2.1 Cyclic voltammetry
A series of cyclic voltammetry curves were obtained at a temperature of 35 °C, a scan rate of 50 mV/s, an ultrasonic frequency of 40 kHz, an ultrasonic power of 0 W and 100 W, a concentration of NH4Cl of 50 g/L, and a Cd2+ concentration of 20 g/L. The test results are shown in Fig. 9.
The cyclic voltammetry curve without ultrasonic coupling in Fig. 9 indicates that line ab is a straight line in the negative potential scan, which indicates the replacement reaction (Formula 1) on the anode surface and the precipitation reaction of Cd (Formula 2) occurs simultaneously. The reaction rate reaches a maximum when the scanning potential is -2.10 V. Then, the current decreases slightly, and the reaction transforms from electrochemical control to diffusion control. At point b, the anodic reaction changes to the hydrogen evolution reaction because the scanning potential reaches -2.40 V. When the potential scans in the opposite direction, the first region is the Cd precipitation reaction, as shown in line cd; that is, Cd2+ receives an electron and is reduced to Cd metal (Formula 1). The second region is line de; when the potential scans in the positive direction, the anode can dissolve a small amount of Zn2+ into the solution. When the potential scans in the negative direction, Zn2+ gains electrons and is reduced to Zn metal (Formula 5). The Zn precipitation rate reaches a peak when the scanning potential is -1.92 V. Subsequently, as the scanning potential gradually increases, the Zn precipitation reaction transforms from diffusion control to electrochemical control. Line eg is the core reaction region of the whole replacement reaction. The main equation is shown in Formula 2. When the scanning potential is -0.86 V, the current is 0 A, indicating that the voltage of the spontaneous replacement reaction is -0.86 V. When the scanning potential is 0 V, the corresponding current is -0.19 A, indicating that this reaction is a spontaneous reaction. The current decreases at point g (0.96 V), and the reaction continues to change to the dissolution reaction of Zn (Formula 3). The effects of the applied current on the extraction of cadmium by electrically enhanced replacement without ultrasonic coupling can be explained by the cyclic voltammetry curve. The reaction (Formula 2) is a spontaneous reaction carried out without applying an external current. The terminal voltage of the spontaneous replacement reaction is -0.86 V, indicating that the reaction is still a replacement reaction when the voltage is between 0.96 and 0 V. The appropriate increase in voltage can accelerate the electrochemical reaction and facilitate the production of Cd.
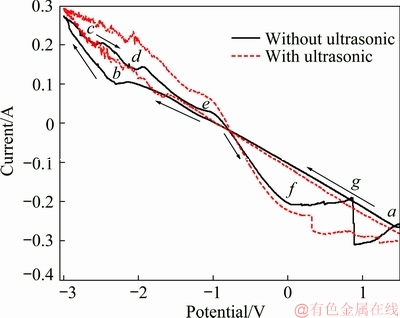
Fig. 9 Cyclic voltammetry curves with and without ultrasonic coupling
The cyclic voltammetry curve with ultrasonic coupling indicates that the ultrasonic coupling blurs the boundaries of each reaction. As the potential scans from negative to positive, the reaction remains the same as that without ultrasonic coupling. However, it can be seen that the slope of the corresponding line is obviously larger than that without ultrasound. The increase in the slope indicates that the accelerated movement of ions in the solution leads to a decrease in the resistance in solution, increasing the reaction rate. The hydrogen evolution reaction occurs with a scanning potential of -2.00 V. Compared to the hydrogen evolution reaction that occurred at -2.40 V without ultrasonic coupling, the potential obviously decreases, indicating that ultrasound can reduce the potential required for the hydrogen evolution reaction. When the potential scans in the negative direction, four reactions occur: Cd2+ accepts electrons to form Cd, Zn2+ accepts electrons to form Zn, Zn replaces Cd2+, and Zn dissolves. The replacement reaction occurs spontaneously with a terminal voltage of -0.86 V. However, the voltage of the zinc dissolution initial reaction decreases from 0.95 to 0.35 V, which indicates that the voltage range of the electrically enhanced replacement reaction with ultrasonic coupling is 0.35 V > Uterminal > 0 V. An applied voltage in this range can promote and accelerate the replacement reaction. In addition, the decrease in the initial potential of the zinc dissolution reaction also indicates that ultrasonic coupling can accelerate the replacement reaction.
Cd2++2e=Cd (1)
Cd2++Zn=Zn2++Cd (2)
Zn-2e=Zn2+ (3)
2H++2e=H2 (4)
Zn2++2e=Zn (5)
3.2.2 Linear polarization
To further test and compare the effects on cadmium extraction under electrically enhanced replacement with or without ultrasonic coupling, the anodic polarization curves were obtained at temperatures of 25 to 45 °C with an ultrasonic power from 0 to 100 W by linear sweep voltammetry.
(1) The effect of temperature on electrically enhanced replacement of cadmium under ultrasonic coupling conditions was investigated.
A series of anodic polarization curves were obtained at temperatures of 25, 30, 35, 40 and 45 °C with a scanning rate of 50 mV/s, a concentration of NH4Cl of 50 g/L, a Cd2+ concentration of 20 g/L, and an ultrasonic power of 0 W. The test results are shown in Fig. 10. The initial potential of the reaction between Zn and Cd2+ in solution does not change with increasing temperature and is approximately -1.08 V. The reason why the initial potential decreases compared to that in Fig. 9 is that when the scanning potential goes from positive to negative, a small amount of cadmium is generated on the working electrode. The reaction changes from Cd dissolution to Cd replacement, but the Cd dissolution reaction does not occur in linear sweep voltammetry. However, the slope of the line increases gradually with the increasing reaction temperature, indicating that the temperature can change the active degree of conductive ions in the solution. The higher the temperature is, the higher the active degree of conductive ions in the solution. The lower the total resistance of the solution is, the faster the reaction rate is. Therefore, a proper increase in temperature is conducive to the replacement reaction and can accelerate the reaction rate.
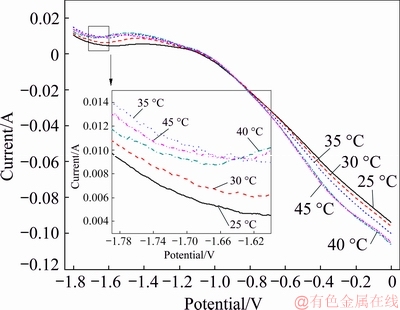
Fig. 10 Anodic polarization curves at different temperatures without ultrasonic coupling
A series of anodic polarization curves were obtained at temperatures of 25, 30, 35, 40 and 45 °C with a scanning rate of 50 mV/s, an ultrasonic frequency of 40 kHz, a concentration of NH4Cl of 50 g/L, a Cd2+ concentration of 20 g/L, and an ultrasonic power of 100 W. Figure 11 shows that the spontaneous reaction occurs more easily with increasing temperature. Compared with the polarization curves without ultrasonic coupling, the inflection point disappears in the curves, and the curves are nearly straight lines. Because the concentration polarization in the solution is reduced, the Cd2+ ions required around the working electrode are always sufficient when ultrasonic coupling exists. The current cannot have an inflection point due to a sudden decrease in Cd2+ around the working electrode, and the replacement reaction will not slow down correspondingly. At the same time, the amplitude of the current variation with ultrasonic coupling is obviously larger than that without ultrasonic coupling. This change indicates that ultrasonic coupling speeds up the reaction, and the acceleration of the reaction rate by ultrasonic coupling is much more obvious than that observed with temperature.
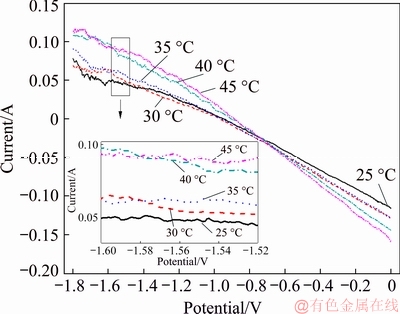
Fig. 11 Anodic polarization curves at different temperatures with ultrasonic coupling
(2) The effect of ultrasonic power on electrical replacement of cadmium under ultrasonic coupling conditions was investigated. A series of anodic polarization curves were obtained under an ultrasonic power of 0, 20, 40, 60, 80 and 100 W with an ultrasonic frequency of 40 kHz, a scan rate of 50 mV/s, a temperature of 25 °C, a concentration of NH4Cl of 50 g/L and a Cd2+ concentration of 20 g/L. The test results are shown in Fig. 12. Figure 12 shows that the application of ultrasound has a great impact on the curves. The curves remain basically flat when the ultrasonic power is 0 W as the scanning potential moves from -1.8 to -1.0 V. The reason for the flat curves is that the potential cannot reach the potential required to reduce Cd2+ to Cd, and the potential is too negative for the substitution reaction to occur spontaneously. The curve is basically a straight line when the potential scans to -1.05 V. The current is 0 A when the scanning potential is -1.08 V, indicating that the initial potential of the spontaneous reaction is -1.08 V. When the ultrasound is applied, the flat line at the front of the curve disappears, indicating that the reaction continues. At the same time, the left end of the curves has a steeper slope with increasing ultrasonic power, that is, with increasing current, which indicates that the larger the ultrasonic power is, the more intense the reaction is. When the current is 0 A, ultrasonic powers of 0, 20, 40, 60, 80 and 100 W result in potentials of -1.05, -1.01, -1.00, -1.00 and -0.95 V, respectively. It shows that the higher the ultrasonic power is, the closer the potential required for the reaction converging towards zero potential is, which means that the reaction can occur easily.
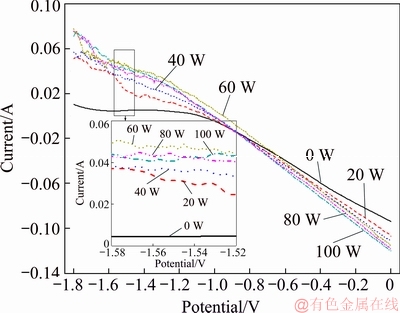
Fig. 12 Anodic polarization curves under different ultrasonic powers
4 Conclusions
(1) The optimum conditions for the extraction of cadmium by electrically enhanced replacement from zinc ammoniacal system were: temperature of 35 °C, cathode- to-anode area ratio of 1:2, anode current density of 15 A/m2, reaction time of 6 h. The replacement rate of cadmium under these conditions was 96.68%. The replacement rate of cadmium increased to 99.21% with an ultrasonic power and frequency of 100 W and 40 kHz.
(2) The ultrasonic coupling with cadmium electrically enhanced replacement in the ammoniacal system improved the replacement efficiency, promoted the separation of cadmium attached to the anode surface, reduced the formation of gas, and inhibited the production of “floating sponge cadmium”.
(3) Ultrasonic coupling decreased the concentration polarization of the solution and reduced the region of the electrically enhanced replacement reaction potential. Without ultrasonic coupling, the range of the replacement reaction potential was between -1.08 and 0.96 V, and the range of the replacement reaction potential was between -0.95 and 0.35 V with ultrasound. Additionally, with increasing temperature, the reaction rate increased. The accelerated movement of ions in the solution reduced the resistance of the solution in the ultrasonic field. Electrochemical control is the main control step of this reaction.
References
[1] ZHAO Tian-cong. Heavy metals metallurgy [M]. Beijing: Metallurgy Industry Press, 1981: 362. (in Chinese)
[2] SAFARZADEH M S, BAFGHI M S, MORADKHANI D, IIKHCHI M O. A review on hydrometallurgical extraction and recovery of cadmium from various resources [J]. Minerals Engineering, 2007, 20(3): 211-220.
[3] SHAO Qiong, DU Xia, WANG Ling, LAN Yao-zhong. Present status of reutilization of copper-cadmium slag [J]. Hydrometallurgy of China, 2003, 22(2): 66-68. (in Chinese)
[4] DAI Shi-ming, LU Xi-wu, Advances on cadmium pollution water treatment technology [J]. Safety and Environmental Engineering, 2006, 13(3): 64-65. (in Chinese)
[5] ZOU Xiao-ping, WANG Sheng-dong, JIANG Xun-xiong, JIANG Ying-ping, WANG Hai-bei, LIU San-ping. Process optimization research of extracting cadmium sponge from copper and cadmium residue [J]. Nonferrous Metals (Extractive Metallurgy), 2010(6): 2-5. (in Chinese)
[6] YANG Jian-guang, FANG Xi, CHAI Li-yuan, MIN Xiao-bo, LI Ling-Chen, YAN Wan-peng, DING Long, NAN Tian-xiang. A method of recovering cadmium from metallurgical dust containing cadmium and a device for recovering cadmium from cadmium- ammonia solution: Chinese patent, CN108220999A [P]. 2018-06-29. (in Chinese)
[7] GOUVEA L R, MORAIS C A. Recovery of zinc and cadmium from industrial waste by leaching/cementation [J]. Minerals Engineering, 2007, 20(9): 956-958.
[8] XU Wen-jie, GUO Qing-wei, XU Zhen-cheng, WANG Yan-fei, ZHOU Qiong-fang, YI Hao. Performance of cadmium waste water treatment by electrodeposition [J]. Water Pollution Control, 2014: 23-26. (in Chinese)
[9] XU Meng, PEJMAN H, CHEN Guo-hua, GORDON M. Removal of cadmium ions from wastewater using innovative electronic waste-derived material [J]. Journal of Hazardous Materials, 2014, 273: 118-123.
[10] LU Dian-kun, JIN Zhen-an, SHI Li-fang, TU Gan-feng, XIE Feng, EDOUARD A. A novel separation process for detoxifying cadmium- containing residues from zinc purification plants [J]. Minerals Engineering, 2014, 64: 1-6. (in Chinese)
[11] HE Jing, QI Chun-ping, DUAN Liang-hong, YE Long-gang, ZHAO Jia-wei, WANG Xia-yang. Process optimization and electrochemistry study of electrical replacement for cadmium extraction based on orthogonal experiments [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(4): 1063-1071. (in Chinese)
[12] YANG Jian-guang, LEI Jie, PENG Si-yao, HE Jing, QI Chun-ping, WANG Xia-yang, LI Jun-yuan. Extraction of cadmium from leaching solution of Cu-Cd slag through electrically enhanced replacement process [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(8): 2268-2275. (in Chinese)
[13] YANG Jian-guang, ZHENG Shi-li, HE Jing, ZHANG Ying, TANG Chao-bo, YANG Sheng-hai, CHEN Yong-ming. A method of recovering copper and cadmium from Cu-Cd slag and apparatus for recovering cadmium from the rich cadmium zinc sulfate solution: Chinese patent, CN103556180A [P]. 2014-02-05. (in Chinese)
[14] ROSE J L. Ultrasonic waves in solid media [J]. Journal of the Acoustical Society of America, 2000, 107(4): 1807-1808.
[15] CHOI Y, HWANG T M, JEONG S, LEE S. The use of ultrasound to reduce internal concentration polarization in forward osmosis [J]. Ultrasonics Sonochemistry, 2018, 41: 475-483.
[16] MILLER D L, PISLARU S V, GREENLEAF J F. Sonoporation: Mechanical DNA delivery by ultrasonic cavitation [J]. Somatic Cell & Molecular Genetics, 2002, 27(1-6): 115-134.
[17] DESAI S A, ROLSTON J D, GUO L, POTTER S M. Improving impedance of implantable microwire multi-electrode arrays by ultrasonic electroplating of durable platinum black [J]. Frontiers in Neuroengineering, 2010, 3(5): 1-11.
[18] HIEDEMANN E A. Metallurgical effects of ultrasonic waves [J]. Journal of the Acoustical Society of America, 1954, 26(5): 831-842.
[19] RASHAD A, SADR E P, WEUSTER M, SCHMITZ I, PROCHNOW N, MAURER P. Material attrition and bone micromorphology after conventional and ultrasonic implant site preparation [J]. Clin Oral Implants Res, 2013, 24(A100): 110-114.
南天翔1,杨建广1,汪文超1,李陵晨1,杨建英2
1. 中南大学 冶金与环境学院,长沙 410083;
2. 江西省环境工程职业学院,赣州 342000
摘 要:采用单因素实验法研究有超声耦合时氨性体系中锌电加强置换提镉的优化工艺条件。结果表明,在温度35 °C、阴阳两极极板面积比1:2、阳极电流密度15 A/m2、耦合超声频率为40 kHz、超声功率100 W条件下以锌板为阳极置换溶液中的镉6 h,镉的置换率为99.21%,且显著抑制“漂浮海绵镉”的产生。采用循环伏安法和线性扫描伏安法,对比研究氨性体系下有/无超声耦合时锌电加强置换提镉的阳极反应机理。循环伏安法表明超声波能促进置换反应的进行,加快置换反应速率,减小电加强置换反应所需电压,改变反应控制步骤;线性扫描伏安法表明,温度及超声功率的增加均能够促进电加强置换反应的进行,加快反应速率,降低置换自发反应所需电位。
关键词:镉;电加强置换;超声波;电化学机理;漂浮海绵镉
(Edited by Xiang-qun LI)
Foundation item: Project (51574294) supported by the National Natural Science Foundation of China; Project (2018zzts447) supported by the Fundamental Research Funds for the Central Universities of Central South University, China
Corresponding author: Jian-guang YANG; Tel: +86-731-88830470; E-mail: jianguang_y@163.com
DOI: 10.1016/S1003-6326(19)65104-6

