合成过程对Al-Ti-B合金中TiB2粒子三维形貌的影响
来源期刊:中国有色金属学报(英文版)2012年第3期
论文作者:李鹏廷 李云国 聂金凤 刘相法
文章页码:564 - 570
关键词:Al-Ti-B合金;TiB2;合成过程;三维形貌
Key words:Al-Ti-B alloy; TiB2; forming process; three-dimensional morphology

![]()

Trans. Nonferrous Met. Soc. China 22(2012) 564-570
LI Peng-ting, LI Yun-guo, NIE Jin-feng, LIU Xiang-fa
Key Laboratory for Liquid-Solid Structural Evolution and Processing of Materials,
Ministry of Education, Shandong University, Ji’nan 250061, China
Received 21 March 2011; accepted 20 December 2011
Abstract: A series of Al-Ti-B master alloys were prepared by different preparation routes, and the TiB2 particles in the master alloys were extracted and analyzed. It is found that the forming process has significant influence on the three-dimensional morphology of TiB2 particles. Different preparation routes result in different reaction forms, which accounts for the morphology variation of TiB2 particles. When the Al-Ti-B master alloy is prepared using “halide salt” route, TiB2 particles exhibit hexagonal platelet morphology and are independent with each other. In addition, the reaction temperature almost does not have influence on the morphology of TiB2 particles. However, TiB2 particles exhibit different morphologies at different reaction temperatures when the master alloys are prepared with Al-3B and Ti sponge. When the master alloy is prepared at 850 °C, a kind of TiB2 particle agglomeration forms with a size larger than 5 μm. The TiB2 particles change to layered stacking morphology even dendritic morphology with the reaction temperature reaching up to 1200 °C.
Key words: Al-Ti-B alloy; TiB2; forming process; three-dimensional morphology
1 Introduction
Al-Ti-B master alloys are good refiners for Al and its alloys [1-3], and Al-TiB2 alloys can be used as composites due to good wear resistance, high tensile strength and elastic modulus [4-6]. The widely used preparation method of Al-Ti-B alloys is “halide salt” route [5-8], but it suffers several drawbacks in practice caused by the low content of B and Ti in the fluoride, such as a large amount of fluoride addition, serious pollution, and difficulty to control [2,9,10]. In recent years, extensive studies have been conducted by changing the raw materials and process. For example, Y?CEL [11] prepared a more effective Al-Ti-B master alloy by replacing KBF4 with Na2B4O7 or B2O3 via self-propagating high-temperature synthesis (SHS) technology.
From existing studies, it is found that different preparation routes result in different refining abilities or mechanical properties of the Al-Ti-B alloys [2, 7-13]. It is thought that the morphology of TiB2 particles plays a vital role in the grain refiners and composites. However, the studies of Al-Ti-B alloy were mainly focused on the morphology of TiAl3 particles and the distribution of TiB2 particles in previous works [7-11], and a comprehensive study on the three-dimensional morphology of TiB2 particles in Al-Ti-B alloy has not been conducted.
In this study, Al-5Ti-1B (all compositions quoted in this work are in mass fraction unless otherwise stated) master alloys were prepared by different processes, and the TiB2 particles in the master alloys were extracted. The influence of forming process on the three- dimensional morphology of TiB2 particles in Al-Ti-B master alloys was studied.
2 Experimental
A series of Al-5Ti-1B master alloys were prepared by different processes (Table 1). 99% KBF4 and K2TiF6, 99.8% Ti sponge, 99.7% commercial pure Al and Al-3B master alloy were used in this study. About 2 kg commercial pure Al was melted in a medium frequency furnace and the mixed powders of KBF4 and K2TiF6 were added into the melt at 850 °C or 1200 °C, which were designated as S1 and S2, respectively. After holding for 10 min, the melt was poured into an iron mould (50 mm in diameter and 25 mm in height). Another two Al-5Ti-1B master alloys were prepared with Al-3B and Ti sponge at 850 °C or 1200 °C, and were designated as S3 and S4 respectively.
Table 1 Preparation of experimental master alloys

The TB2 particles were extracted from all the master alloys by a special extraction method to observe the three-dimensional morphology [14]. First, the Al matrix was dissolved in a solution of 10% HCl. Next, the particles in the solution were centrifuged by a centrifugal extractor. And then the collected sediments were rinsed with distilled water and ethanol several times. The metallographic specimens were intercepted from each bottom of the samples and the phase constituent was identified by X-ray diffraction (XRD). The microstructure of the alloy and the particle morphology were examined on a field emission scanning electron microscope (FESEM) equipped with an energy- dispersive spectroscopy detector (EDS).
3 Results and discussion
3.1 Microstructure of master alloys
Figure 1 shows the microstructure of the Al-5Ti-1B master alloys prepared by “halide salt” route (S1 and S2). It can be seen that the second phase particles are relatively dispersed in Al matrix in samples S1 and S2. Furthermore, there are some block-like TiAl3 in sample S1, as shown in Fig. 1(a). In sample S2, TiAl3 shows stick-like morphology and is larger than that in sample S1 (Fig. 1(b)), which is attributed to the higher melting and pouring temperature.
In order to observe three-dimensional morphology and size of TiB2 particles, the particles were extracted from the master alloys. Figures 2(a) and (b) show the morphologies of TiB2 particles extracted from sample S1 and S2, respectively. From the corresponding XRD results in Fig. 2(a) it is found the particles mainly contain two kinds of phases: TiB2 and TiAl3. The morphology of TiB2 particles in sample S1 is hexagonal platelet, and the size is about 1 μm (with the thickness of 0.2-0.5 μm). And TiB2 particles are independent with each other. The particles in sample S2 also exhibit hexagonal platelet morphology but its size is variable, which is different with that in sample S1. Some TiB2 particles are about 2 μm in size while others are smaller than 1 μm. The morphology of TiB2 particles in samples S1 and S2 are almost the same, so it can be concluded that reaction temperature does not affect the morphology of TiB2 particles in the Al-5Ti-1B master alloys prepared by “halide salt” route.

Fig. 1 FESEM images of Al-5Ti-1B master alloys prepared by “halide salt” route: (a) Sample S1; (b) Sample S2
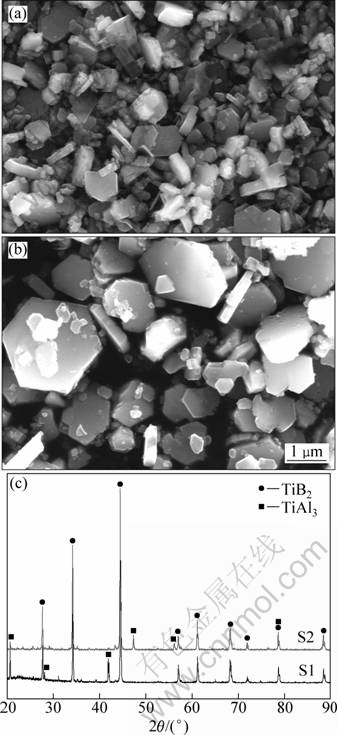
Fig. 2 FESEM images of particles extracted from samples S1 (a) and S2 (b) and XRD patterns (c)
Figure 3(a) shows the SEM image of sample S3. The distribution and morphologies of TiB2 are diverse. Some of TiB2 are particle-like and dispersed in the Al matrix, which are similar to those in samples S1 and S2. Moreover, a lot of TiB2 particles connect each other to form chain-like structures or agglomerate together. A TiB2 agglomeration is magnified and analyzed by EDS, as shown in Figs. 3(b), (c) and (d). It can be seen that, in this agglomeration, the TiB2 particles closely agglomerate and connect each other to form a whole. In the center of the TiB2 agglomerations, unreacted AlB2 particles are observed. The typical morphology of TiB2 particles in sample S3 is shown in Fig. 4. The TiB2 particles in sample S3 exist in the form of agglomeration with a size larger than 5 μm, which has been mentioned as dense agglomeration in some articles [15-18]. It is difficult for the agglomerative particles to spread in the Al melt during refinement.
Figure 5 shows the SEM image of sample S4 and the corresponding XRD result. The master alloy mainly contains α(Al), TiAl3 and TiB2 phases. From the microstructure of the alloy shown in Fig. 5(b), it can be seen that TiAl3 particles show coarse stick-like morphology, and the distribution of TiB2 particles also changes with the increase of the reaction temperature. Particles in sample S4 are extracted for better observation, and Fig. 6 shows the typical morphologies of TiB2 particles. TiB2 particles exhibit layered stacking (Fig. 6(a)) and dendritic (Fig. 6(b)) morphologies, rather than independent hexagonal platelet or agglomeration. The layered stacking TiB2 particle in Fig. 6(a) is composed of multiple thin platelets that are smaller than 0.1 μm in thickness. There are displacements between neighboring thin platelets, which helps to form a ladder structure along a certain direction [18]. The TiB2 particle in Fig. 6(b) exhibits the dendritic morphology which is more complex than the layered stacking morphology. It can be seen that each branch of the dendritic TiB2 particles is also layered stacking structure.
3.2 Discussion
TiB2 crystallizes in a hexagonal lattice P6/mmm. It is characterized by alternating hexagonal layers of Ti and B atoms [19]. In the hexagonal structure of TiB2, {0001} planes share the highest surface atomic density, which also have the lowest surface energy and the slowest growth rate. Their crystal development will be limited and the face will be shown up at last [20]. So, the TiB2particles would exhibit hexagonal morphology features. However, the three-dimensional morphologies of the TiB2 particles in samples S1, S2, S3, and S4 are not completely identical, as shown in the results above. It is thought that this difference in particle morphology is caused by the different reactions occurring in samples S1, S2, S3, and S4 due to the different forming processes.
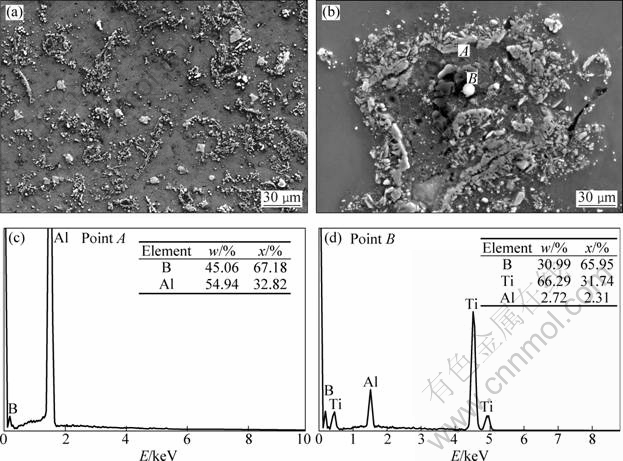
Fig. 3 FESEM images of sample S3 (a, b) and corresponding EDS results (c, d)
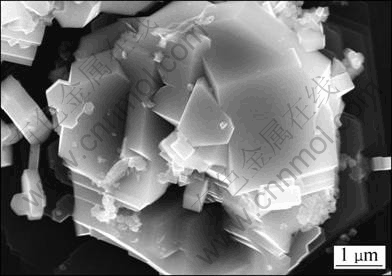
Fig. 4 FESEM image of TiB2 agglomeration extracted from sample S3
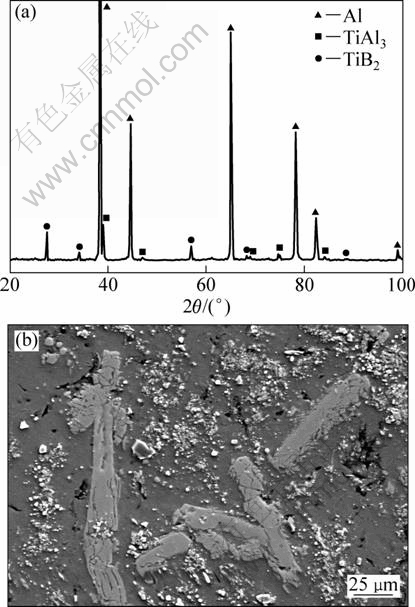
Fig. 5 XRD pattern (a) and FESEM image (b) of sample S4
The formation of TiB2 particles has been studied by many researchers [13,16]. The possible reactions in this study are
3K2TiF6(l)+2KBF4(l)+12Al(l) →2TiAl3(s)+TiB2(s)+5KAlF4(l)+K3AlF6(l) (1)
AlB2(s)+Ti→TiB2(s)+Al(l) (2)
2B+Ti→TiB2(s) (3)
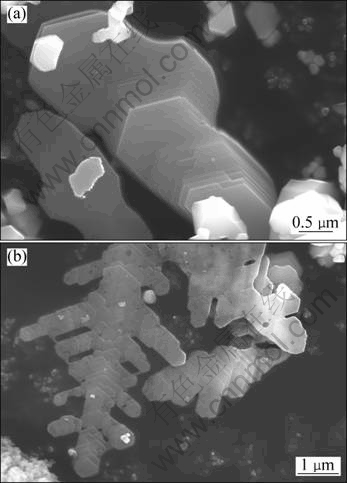
Fig. 6 SEM images showing typical morphologies of layered stacking (a) and dendritic TiB2 particles (b) in sample S4
where B and Ti are solutes and the AlB2(s) can be added by Al-B alloy or formed in the melt.
Reaction (1) is the main reaction occurring in the preparation of Al-Ti-B alloy by the “halide salt” route. When the mixed powders of KBF4 and K2TiF6 are added during the preparations of S1 and S2, they float in the upper of the melt because of the low density. Reaction (1) mainly takes place in a high rate in the interface region between the mixed powders and the Al melt [17]. Figure 7(a) shows the schematic illustration of the fluoride salt/Al interfacial reaction. It should be pointed out that the concentrations of B and Ti atoms in the interface region are relatively high, which is helpful for the nucleation of TiB2 particles. So, the nucleation of TiB2 is intensive and many particles form in a very short time [21]. TiB2 particles with the hexagonal morphology will form as a result of the reaction in the interface region, and fall down to the bottom of the melt when particles grow to some extent [22]. The growth of TiB2 particles will stop after the fall. So, the TiB2 particles in samples S1 and S2 exhibit the same morphology. It also indicates that the reaction temperature has little influence on the morphology of the TiB2 particles in reaction (1). However, as shown in Fig. 2, the size of TiB2 particles in sample S2 changes. Some TiB2 particles become more than 2 μm in size while others are smaller than 1 μm. TONG et al [23] pointed out that dissolution of second phase particles in a high-temperature Al melt will occur to a certain extent, and the small particles are more likely to dissolve than big particles. It means the smallest TiB2 particles nucleated in situ-reaction process re-dissolve into the high-temperature melt, and the amount of solute B is large so that the melt may be over-saturated during cooling process. It is much easier for solute Ti and B to precipitate on the surface of nucleated TiB2 particles, because heterogeneous nucleation acquires less nucleation energy and lower undercooling. Therefore, the TiB2 particles can grow larger during the solidification process. However, with the decrease of temperature, mass transport is getting more and more difficult as a result of the decrease of diffusion coefficient [24]. In some zone of the low temperature melt where solutes Ti and B are not able to diffuse to the surface of nucleated TiB2 particles, they dissolve out as micro size TiB2 particles. As a result, the size of the TiB2 particles in sample S2 is variable due to the dissolution and precipitation of some TiB2 particles at high temperature.
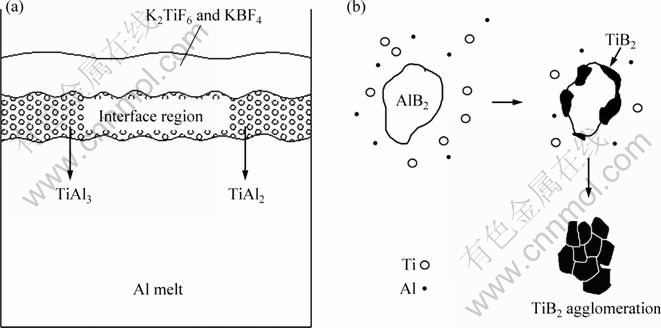
Fig. 7 Schematic illustrations of fluoride salt/Al interfacial reaction (a) and formation of TiB2 particle agglomeration (b) in sample S3
When the Al-Ti-B alloys are prepared using Al-3B and Ti sponge, the reaction forms change. So, the morphologies of the TiB2 particles in samples S3 and S4 also change. As shown in Fig. 4, the TiB2 particles in sample S3 tend to be in agglomeration. It is believed that the formation of TiB2 agglomeration is related to the formation of B-containing compound in the reaction. Some articles [25,26] pointed out that the solubility of B in Al melt is very small at 850 °C and B mainly exists in the form of AlB2 phase. So, it is thus proposed that TiB2 in sample S3 forms through a reaction between solutes Ti and AlB2 (reaction (2)). The schematic illustration of the agglomeration formation is shown in Fig. 7(b) and the mechanism is offered as follows: when Ti sponge is added, it starts to dissolve in molten Al and the solute Ti reacts with the existing AlB2 to form the TiB2 phase by replacing the Al atoms in the AlB2 particles gradually. With the reaction process going, some TiB2 particles form on the surface of AlB2 particles, and they link with each other closely in the subsequent growth process. TiAl3 particles start to precipitate in the melt only when AlB2 fully reacts and the Ti solubility limit (approximately 1.0 % at about 850 °C) is exceeded.
However, if the experiment temperature is raised to 1200 °C, the AlB2 particles from Al-3B master alloy will dissolve. So, the solute Ti will preferentially react with B to form the TiB2 phase during the preparation of S4, which can be expressed by reaction (3). It should be pointed out that the concentration of B atoms is low because they have dispersed in the whole melt. Under such condition, the successive growth of the TiB2 particles is easier than particle nucleation. During the reaction process, thin hexagonal platelets form in the melt firstly. With the reduction in the concentration of atoms B and Ti, the nucleation of TiB2 particles becomes more and more difficult. So, the surrounding B and Ti atoms will deposit on the surface of the nucleated particles, and new plates will form on the surface of the formed thin hexagonal platelets. On the other hand, some growth steps in TiB2 particles emerge on the {0001} planes, as shown in Fig. 6. The presence of these growth steps indicates that the two-dimensional nucleation growth of the {0001} planes is favorable [27, 28]. This is possibly attributed to the higher reaction rate and atomic diffusion rate when the reaction temperature increases, leading to a higher density of defects (such as impurities and dislocations) in the TiB2 crystal structure [28]. It implies that there will be more steps created by crystal defects for the growth unit to deposit on the {0001} planes. As a result, with the deposition of atoms B and Ti and the new steps generating, the TiB2 particles with layered stacking even dendritic structure form in the Al melt finally.
The primary factor influencing the TiB2 morphology can be ascribed to the forming process because it is most noticeable and easy to understand. More profound investigations on the TiB2 crystal growth are still in progress.
4 Conclusions
1) The forming process has significant influence on the three-dimensional morphology of TiB2 particles in Al-Ti-B master alloys. Different preparation routes result in different reactions, which accounts for the morphology variation of TiB2 particles.
2) TiB2 particles exhibit hexagonal platelet morphology and are independent with each other, when the master alloy is prepared using “halide salt” route. In addition, the reaction temperature has no influence on the morphology of TiB2 particles.
3) TiB2 particles exhibit different morphologies at different reaction temperatures when the master alloys are prepared with Al-3B and Ti sponge. A kind of TiB2 particle agglomeration forms with a size larger than 5 μm at 850 °C. The TiB2 particles change to layered stacking morphology even dendritic morphology with the reaction temperature reaching up to 1200 °C.
References
[1] MOHANTY P S, GRUZLESKI J E. Mechanism of grain refinement in aluminium [J]. Acta Metallurgica et Materialia, 1995, 43: 2001-2012.
[2] KRISHNANA T S, RAJAGOPALANA P K, CUNDB B R, BOSEC D K. Development of Al-5%Ti-1%B master alloy [J]. Journal of Alloys and Compounds, 1998, 269: 138-140.
[3] EASTON M A, STJOHN D H. A model of grain refinement incorporating alloy constitution and potency of heterogeneous nucleant particles [J]. Acta Materialia, 2001, 49: 1867-1878.
[4] WILLIAM C. Commercial processing of metal matrix composites [J]. Materials Science and Engineering A, 1998, 24: 75-79.
[5] YI H Z, MA N H, ZHANG Y J, LI X F, WANG H W. Effective elastic moduli of Al-Si composites reinforced in situ with TiB2 particles [J]. Scripta Materialia, 2006, 54: 1093-1097.
[6] TEE K L, LU L, LAI M O. In situ processing of Al-TiB2 composite by the stir-casting technique [J]. Journal of Alloys and Compounds, 1999, 89-90: 513-519.
[7] MURTI B S, KORI S A, VENKATESWARLU K, BHAT R R, CHAKRABORTI M. Manufacture of Al-Ti-B master alloys by the reaction of complex halide salts with molten aluminium [J]. Journal of Materials Processing Technology, 1999, 89-90: 152-158.
[8] HAN Y F, SHU D, WANG J, SUN B D. Microstructure and grain refining performance of Al-5Ti-1B master alloy prepared under high-intensity ultrasound [J]. Materials Science and Engineering A, 2006, 430: 326-331.
[9] XU Chun-xiang, LIANG Li-ping, LU Bin-feng, ZHANG Jin-shan, LIANG Wei. Effect of La on microstructure and gain-refining performance of Al-Ti-C grain refiner [J]. Journal of Rare Earths, 2006, 24: 596-601.
[10] CHEN Ya-jun, XU Qing-yan, HUANG Tian-you. Refining performance and long time efficiency of Al-Ti-B-RE master alloy [J]. The Chinese Journal of Nonferrous Metals, 2007, 17: 1232-1239. (in Chinese)
[11] Y?CEL B. Production of Al-Ti-B grain refining master alloys from B2O3 and K2TiF6 [J]. Journal of Alloys and Compounds, 2007, 443: 94-98.
[12] WANG M X, WANG S J, LIU Z Y, LIU Z X, SONG T F, ZUO X R. Effect of B/Ti mass ratio on grain refining of low-titanium aluminum produced by electrolysis [J]. Materials Science and Engineering A, 2006, 416: 312-316.
[13] YUCEL B. Production of Al-Ti-B master alloys from Ti sponge and KBF4 [J]. Journal of Alloys and Compounds, 2007, 440: 108-112.
[14] LIU C P, PAN F S, WANG W Q. Phase analysis of Al-Mn compounds in the AZ magnesium alloys [J]. Materials Science Forum, 2007, 546-549: 395-398.
[15] SCHAFFER P L, DAHLE A K. Settling behaviour of different grain refiners in aluminium [J]. Materials Science and Engineering A, 2005, 413-414: 373-378.
[16] EMAMY M, MAHTA M, RASIZADEH J. Formation of TiB2 particles during dissolution of TiAl3 in Al-TiB2 metal matrix composite using an in situ technique [J]. Composites Science and Technology, 2006, 66: 1063-1066.
[17] FJELLSTEDT J, JARFORS A E W. On the precipitation of TiB2 in aluminum melts from the reaction with KBF4 and K2TiF6 [J]. Materials Science and Engineering A, 2005, 413-414: 527-532.
[18] LI P T, MA X G, LI Y G, NIE J F, LIU X F. Effects of trace C on the microstructure and refining efficiency of Al-Ti-B master alloy [J]. Journal of Alloys and Compounds, 2010, 503: 286-290.
[19] TIAN D C, WANG X B. Electronic structure and equation of state of TiB2 [J]. Journal of Physics: Condensed Matter, 1992, 4: 8765-8772.
[20] ABDEL-HAMID A A, HAMAR-THIBAULT S, HAMAR R. Crystal morphology of the compound TiB2 [J]. Journal of Crystal Growth, 1985, 71: 744-750.
[21] JHA A, DOMETAKIS C. The dispersion mechanism of TiB2 ceramic phase in molten aluminium and its alloys [J]. Materials and Design, 1997, 18: 297-301.
[22] SCHAFFER P L, ARNBERG L, DAHLE A K. Segregation of particles and its influence on the morphology of the eutectic silicon phase in Al-7 wt.% Si alloys [J]. Scripta Materialia, 2006, 54: 677-682.
[23] TONG X C, FANG H S. Al-TiC composites in situ-processed by ingot metallurgy and rapid solidification technology: Part I. Microstructural evolution [J]. Metallurgical and Materials Transactions A, 1998, 29: 875-891.
[24] WU Q, LI C S, TANG H. Surface characterization and growth mechanism of laminated Ti3SiC2 crystals fabricated by hot isostatic pressing [J]. Applied Surface Science, 2010, 256: 6986-6990.
[25] FJELLSTEDT J, JARFORS A E W, SVENDSEN L. Experimental analysis of the intermediary phases AlB2, AlB12 and TiB2 in the Al-B and Al-Ti-B systems [J]. Journal of Alloys and Compounds, 1999, 283: 192-197.
[26] WANG X M. The formation of AlB2 in an Al-B master alloy [J]. Journal of Alloys and Compounds, 2005, 403: 283-287.
[27] SONG M S, HUANG B, HUO Y Q, ZHANG S G, ZHANG M X, HU Q D, LI J G. Growth of TiC octahedron obtained by self-propagating [J]. Journal of Crystal Growth, 2009, 311: 378-382.
[28] JIN S B, SHEN P, ZOU B L, JIANG Q C. Morphology evolution of TiCx grains during SHS in an Al-Ti-C system [J]. Crystal Growth & Design, 2009, 9: 646-649.
李鹏廷,李云国,聂金凤,刘相法
山东大学 材料液固结构演变与加工教育部重点实验室,济南 250061
摘 要:通过不同工艺制备出一系列Al-Ti-B中间合金,并利用相提取工艺对合金中的TiB2粒子进行提取和分析。研究表明:合成过程对TiB2粒子的三维形貌有显著的影响,不同的制备工艺会导致不同的反应路径,并最终影响TiB2粒子的形貌。利用氟盐法制备的Al-Ti-B中间合金中的TiB2粒子呈现相互独立的六角板块状形貌,且反应温度不会对TiB2粒子的形貌产生影响。然而,利用Al-3B中间合金和海绵Ti制备Al-Ti-B中间合金时,TiB2粒子的形貌随着反应温度的升高而发生明显变化。当反应温度为850 °C时,Al-Ti-B中间合金中的TiB2粒子以大的聚集团形式存在,而当反应温度升高到1200 °C时,TiB2粒子的形貌转变为复杂的层片状和枝晶状。
关键词:Al-Ti-B合金;TiB2;合成过程;三维形貌
(Edited by YANG Hua)
Foundation item: Project (50625101) supported by the National Science Fund for Distinguished Young Scholars of China; Project supported by Graduate Independent Innovation Foundation of Shandong University (GIIFSDU), China; Project (51071097) supported by the National Natural Science Foundation of China
Corresponding author: LIU Xiang-fa; Fax: +86-531-88395414; E-mail: xfliu@sdu.edu.cn
DOI: 10.1016/S1003-6326(11)61214-4

