陶瓷胶态成型用含聚(N-异丙基丙烯酰胺)ZnO温敏悬浮液的流变行为
来源期刊:中国有色金属学报(英文版)2016年第11期
论文作者:孙月花 彭超群 王小锋 王日初 冯艳 王乃光
文章页码:2930 - 2938
关键词:流变行为;ZnO;聚(N-异丙基丙烯酰胺);温敏性;有效固相体积分数
Key words:rheological behavior; ZnO; poly(N-isopropylacrylamide); colloidal processing; thermosensitivity; effective volume fraction
摘 要:采用温敏性聚(N-异丙基丙烯酰胺)(PNIPAM)为凝固剂的新型胶态成型已应用于制备复杂形状的陶瓷器件。本文作者研究PNIPAM水溶液性能和添加PNIPAM的ZnO悬浮液的流变性能。结果表明,PNIPAM表现出明显的温敏性且转变温度在32 °C左右。当温度高于40 °C (Tc,温敏悬浮液的临界转变温度),添加8 mg/mL PNIPAM的50% ZnO(体积分数)悬浮液的黏度急剧增加,温度为50 °C时黏度高达11.49 Pa·s并表现出较强弹 性。原因在于高温下PNIPAM分子的析出导致悬浮液的有效固相体积分数增加以及ZnO陶瓷颗粒之间的絮凝作用。此外,20 °C下悬浮液的最大固相体积分数明显高于40 °C下的,也可证明PNIPAM的相转变可诱导悬浮液的絮凝。
Abstract: Novel colloidal processing using thermosensitive poly(N-isopropylacrylamide) (PNIPAM) as a coagulating agent has been developed to prepare complex-shaped ceramic components. In this work, the properties of PNIPAM aqueous solutions and the rheological behavior of ZnO suspensions with PNIPAM were investigated. The results show that the PNIPAM solutions exhibit obvious thermosensitivity and its transition temperature is around 32 °C. When the temperature is above 40 °C (Tc, the critical transition temperature of thermosensitive suspension), the 50% ZnO (volume fraction) suspension with 8 mg/mL PNIPAM has a sharp increase in viscosity and reaches up to 11.49 Pa·s at 50 °C, displaying strong elasticity. The main reasons are the increase of effective volume fraction attributed to precipitation of PNIPAM segments and the flocculation between ZnO powder particles. In addition, the maximum solid loading (volume fraction) at 20 °C is higher than that at 40 °C, which proves that the phase transition of PNIPAM can induce the flocculation of suspension.
Trans. Nonferrous Met. Soc. China 26(2016) 2930-2938
Yue-hua SUN, Chao-qun PENG, Xiao-feng WANG, Ri-chu WANG, Yan FENG, Nai-guang WANG
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 21 October 2015; accepted 21 April 2016
Abstract: Novel colloidal processing using thermosensitive poly(N-isopropylacrylamide) (PNIPAM) as a coagulating agent has been developed to prepare complex-shaped ceramic components. In this work, the properties of PNIPAM aqueous solutions and the rheological behavior of ZnO suspensions with PNIPAM were investigated. The results show that the PNIPAM solutions exhibit obvious thermosensitivity and its transition temperature is around 32 °C. When the temperature is above 40 °C (Tc, the critical transition temperature of thermosensitive suspension), the 50% ZnO (volume fraction) suspension with 8 mg/mL PNIPAM has a sharp increase in viscosity and reaches up to 11.49 Pa·s at 50 °C, displaying strong elasticity. The main reasons are the increase of effective volume fraction attributed to precipitation of PNIPAM segments and the flocculation between ZnO powder particles. In addition, the maximum solid loading (volume fraction) at 20 °C is higher than that at 40 °C, which proves that the phase transition of PNIPAM can induce the flocculation of suspension.
Key words: rheological behavior; ZnO; poly(N-isopropylacrylamide); colloidal processing; thermosensitivity; effective volume fraction
1 Introduction
To effectively control particle aggregation and minimize defects, many colloidal processing methods (e.g., gel-casting [1-3], slip casting [4-6], direct ink writing [7-9], and direct coagulation casting [10-12]) have been developed to form complex-shaped ceramics. Among which, direct coagulation casting (DCC) is a novel near-net-shape method with the combination of enzyme technology, colloidal chemistry, and ceramic process, as first developed by BALZER et al [10]. In this process, the dense green body can be achieved from suspension by either shifting the pH of suspension towards its isoelectric point (IEP) or increasing the ionic strength of suspension to compress the electric double layer. It has been utilized in the forming of many sorts of ceramics. However, there are still some drawbacks in this process. In the typical enzyme catalysis system, the strength of green body is significantly low and it is difficult to treat in the subsequent processing [13,14]. Moreover, the preparation of slurry needs to be controlled at a low temperature (<5 °C) to avoid the enzyme catalysis reaction before casting [15,16], and the strict control of temperature increases the process complexity and preparation cost. Although, high valence counter-ions system solves the problem of low strength via increasing the ionic strength, e.g., magnesium ion [17,18] and calcium ion [19,20], the process is still difficult to control.
Recently, we have developed a novel colloidal processing using the thermosensitive poly (N-iso- propylacrylamide) (PNIPAM) as a coagulating agent with the aim of forming ceramic components [21]. PNIPAM exhibits a phase transformation in aqueous solution at a certain temperature due to the existence of hydrophilic amide and hydrophobic isopropyl groups, and this transition temperature is called the lower critical solution temperature (LCST) around 32 °C [22-24]. Below the LCST, macromolecular chain of PNIPAM swells in water. When the temperature is higher than the LCST, the loosely arranged macromolecular chain converts into compact spherical design. The advantage of this method is that the consolidation of suspension can be deliberately controlled by temperature. The forming mechanism of this method and the rheological behavior of suspension are unacquainted, although its feasibility for complex-shaped ZnO ceramics has been investigated. The purpose of this work is to clarify the properties of PNIPAM aqueous solution and rheological behavior of ZnO suspension with thermosensitve PNIPAM.
2 Experimental
A commercial high-purity (>99.9%, mass fraction) ZnO powder (Jiangxi Huarun Co., Ltd., Jiangxi, China) with an average particle size of 0.1 μm and a surface area of 4.47 m2/g was adopted. Analytical reagent poly (acrylic acid) (relative molecular mass 5000, Sigma- Aldrich Chemical Co.) was applied as the dispersing agent. The thermosensitive polymer poly(N-isopropyl- acrylamide) (relative molecular mass 19000-30000, Sigma-Aldrich Chemical Co.) was used as a coagulating agent. The ammonia (NH3·H2O) solution (Shanghai Chemical Reagent Co., Ltd., Shanghai, China) was chosen to adjust the pH value of slurries. Distilled water was used for preparing slurries.
The PNIPAM aqueous solutions at pH 10 were prepared by mixing various amounts of PNIPAM and distilled water to study properties of PNIPAM solution at room temperature (25 °C). ZnO suspensions were made by tumbling the ZnO powder, distilled water, 0.8% poly (acrylic acid) (PAA) and different contents of PNIPAM in high-density poly(ethylene) containers along with ZrO2 grinding balls (the diameter of these balls is 10 mm) for 24 h to make suspensions homogenous. In addition, the mass ratio of ZnO powder to grinding ball was 1:1. The whole process of suspension preparation was carried out at room temperature, and the pH of suspension was adjusted to about 10.
The turbidity of aqueous solutions with various amounts of PNIPAM at the pH of 10 was determined by measuring the transmittance of solution at different temperatures using ultraviolet-visible spectrophotometer (Spectrumlab 752S, Shanghai Lengguang Technology Co., Ltd., China). The wavelength of visible light was 560 nm and the transmittance of distilled water at 20 °C was chosen as the standard reference with transmittance of 100%. The rheological behavior of ZnO suspensions was measured using a rotational rheometer (AR2000EX, TA Instruments, America) with a parallel plate (40 mm in diameter). All the viscosity and shear modulus measurements were taken at a shear rate of 1×10 s-1 and a vibration frequency of 1 Hz.
3 Results
3.1 Solution properties of PNIPAM
Figure 1 shows the effect of PNIPAM concentration on temperature dependence of the transmittance at pH 10. The pH of PNIPAM aqueous solutions chosen as 10 is attributed to good dispersibility of PAA in strong alkaline condition for preparing stable suspensions. The results clearly reveal that the LCST of PNIPAM is highly affected by its concentration. If the relative molecular mass of PNIPAM remains constant, the smaller the concentration is, the higher the LCST is. In the case of four aqueous PNIPAM samples, it can be observed that the transmittance of PNIPAM aqueous solution decreases as the temperature increases and exhibits a steep transition at the LCST around 32 °C. The transmittance decreases slightly at first and the values are higher than 99% when the temperature is lower than 30 °C. Thereafter, the aqueous solutions exhibit an obvious decrease in transmittance when the temperature increases from 30 to 35 °C and a steep transition at a certain temperature. Afterwards, the transmittance reaches the minimum values at about 35 °C and remains essentially unchanged. The difference is that the phase transition temperatures of PNIPAM aqueous solutions with various concentrations are different. That is, the LCST of polymer solutions is depressed with increasing polymer concentration. The LCST of aqueous solution with 2 mg/mL PNIPAM is the highest of about 32.5 °C, whereas that with 8 mg/mL PNIPAM is the lowest of about 32 oC. The minimum transmittance of PNIPAM aqueous solution at temperature higher than 35 °C decreases with the increase of polymer concentration. In conclusion, the LCST of polymer PNIPAM is strongly affected by the concentration, which is consistent with other reports [23,25].
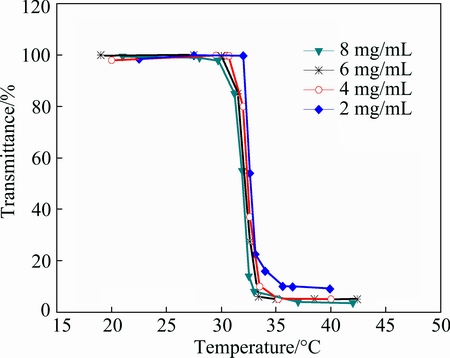
Fig. 1 Transmittance (ratio of intensities of transmitted light and incidence light) of PNIPAM aqueous solution at various concentrations as function of temperature at pH 10
3.2 Rheological behavior of ZnO suspension
Figure 2 shows the effect of temperature on the viscosity of ZnO suspension prepared with 0.8% PAA and different contents of PNIPAM at pH 10. It is observed that the viscosity of suspension increases as the temperature or concentration increases. The viscosity of suspension without PNIPAM is small even when the temperature reaches 50 °C. In contrast, the viscosity of suspensions containing PNIPAM in the range of 2-8 mg/mL increases distinctly as the temperature increases. The results clearly reveal that the viscosity of ZnO suspensions with PNIPAM remains largely unchanged until the temperature is higher than 40 °C. Hence, the critical transition temperature (Tc) of thermosensitive suspension is around 40 °C. It is important to note that the critical transition temperature of thermosentive suspension with PNIPAM is higher than the LCST of PNIPAM (~32 °C). This might be because the presence of suspended ZnO powder particles hinders the formation of tight spherical-state PNIPAM segments through diffusion, which would increase the required thermal activation energy. When the temperature is lower than 40 °C, the influence of temperature on viscosity is less significant. However, the viscosity of suspensions with PNIPAM, especially that with 6 and 8 mg/mL PNIPAM, increases sharply at the temperature above 40 °C, and reaches up to 6.15 and 11.49 Pa·s at 50 °C, respectively, which are high enough to meet the requirements of ceramic forming. Therefore, the rheological transition of thermosensitive ZnO suspensions occurs around Tc rather than the LCST of PNIPAM, which indicates that there is a time- dependence of the rheological behavior in suspensions in addition to the temperature-dependence.

Fig. 2 Effect of temperature on viscosity of 50% ZnO suspension with various concentrations of PNIPAM at pH 10
The steady shear behavior of the concentrated ZnO suspension prepared with 0.8% PAA and 8 mg/mL PNIPAM is presented in Fig. 3. The shear thinning behavior of the suspension can be observed from these curves. That is, the viscosity is not a constant but a variable which decreases when the non-Newtonian fluid endures shear force [26,27]. Figure 3(a) shows the viscosities of ZnO suspensions loading in the range of
35%-50% (volume fraction) with 8 mg/mL PNIPAM at 40 °C and various shear rates. As expected, both viscosity and shear thinning behaviors of the suspensions increase with the increase of solid loading. Besides, the viscosity of ZnO suspension with the solid loading of 50% has the most obvious change under shearing stress and shows flow property suitable for casting. In addition, its viscosity decreases from 509.2 to 0.16 Pa·s as the shear rate increases from 1×10-3 to 1×103 s-1. Figure 3(b) shows the viscosities at various shear rates of ZnO slurries loading in the range of 35%-50% with 8 mg/mL PNIPAM at 20 °C. The ZnO suspension with the solid loading of 50% shows viscosity ranging from 101.8 to 0.13 Pa·s at the shear rates of 1×10-3 to 1×103 s-1, whereas the viscosity of 35% ZnO suspension only changes from 2.58 to 0.02 Pa·s. The results clearly reveal that the suspension with a higher solid loading displays a relatively significant shear thinning behavior approaching a constant at a high shear rate, signifying that the steady shear behavior is highly related to the solid loading. As shown in Figs. 3(a) and (b), it is evident that the shear thinning behavior of the suspension at 40 °C is more remarkable than that at 20 °C under the same solid loading. Therefore, the steady shear is related to both solid loading and temperature.
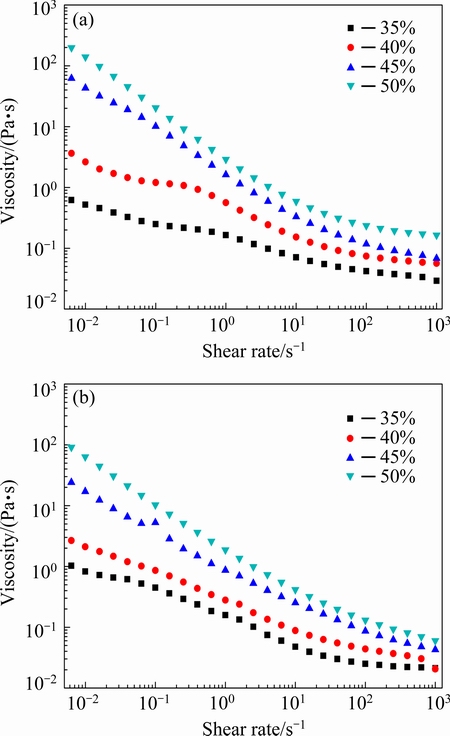
Fig. 3 Viscosity as function of shear rate and solid loading (volume fraction) for ZnO suspensions with 8 mg/mL PNIPAM at 40 °C (a) and 20 °C (b)
The viscoelasticity can reflect the elastic and viscous properties of suspension during the solidification process. Figure 4 shows the storage modulus of 50% ZnO suspension at different temperatures. This frequency sweep measurements are performed at strain of 0.4% which is in the linear viscoelastic range. The changes of storage modulus at 20 and 25 °C are similar and the colloidal property of suspension is dominant. That is, the storage modulus of suspension continuously increases as the angular frequency increases from 1×10-1 to 1×103 rad/s, indicating that the suspension deforms plastically. However, at higher temperatures (i.e., 33, 35, and 40 °C), the storage modulus of suspension increases first and then remains constant as the angular frequency increases, revealing that the suspension begins to deform elastically. The 50% ZnO suspension with 8 mg/mL PNIPAM is gel-like below Tc, while this suspension is solid-like above Tc. Thus, the ZnO suspension changes from being predominately viscous to displaying a strongly elastic behavior as the temperature increases.

Fig. 4 Storage modulus (G′) of 50% ZnO suspension with 8 mg/mL PNIPAM as function of temperature and angle frequency ω
4 Discussion
The PNIPAM is a typical temperature responsive polymer, as characterized by the LCST. The LCST phenomenon existing in PNIPAM aqueous solution should be interpreted as a result of the phase transformation. At the temperature lower than the LCST, the hydrogen bonding among water molecules and hydrophilic acylamino groups of PNIPAM polymer is dominant, the hydrophobic interaction evokes the rearrangement of water molecules to form well-ordered solvation layers around the hydrophobic isopropyl groups due to the effect of hydrogen bonding and van der Waals force. PNIPAM dissolving in water forms random coiled architecture [28]. Thus, the transmittance of aqueous PNIPAM solution is significantly higher at a low temperature. When the temperature is higher than the LCST, a part of hydrogen bonding is destroyed, leading to the breakdown of solvation layers surrounding the hydrophobic segments. The formative hydrophobic layers force the water molecules to release from the solvation layer owing to the enhancement of hydrophobic interaction, and convert the loose coiled conformation into the tight colloidal structure. Therefore, the PNIPAM aqueous solution becomes turbid and its transmittance decreases sharply as the temperature increases due to the suspended colloidal particles. In addition, Fig. 1 reveals that high concentration of PNIPAM depresses the value of the phase transition temperature. The study of PAMIES et al [25] indicates that this phenomenon can be ascribed to stronger intermolecular association at higher concentration, leading to a reduction of lower critical solution temperature. The PNIPAM aqueous solution with high concentration contains a large number of isopropyl groups, which displays more intense hydrophobic character at higher temperature. In addition, the increase of formative colloidal particles in solution with increasing PNIPAM concentration leads to the lower transmittance of high polymer concentration at the temperature higher than the LCST. Thus, it can be concluded that the LCST of aqueous PNIPAM solution is around 32 °C, which is consistent with the LCST reported by other researchers [28,29].
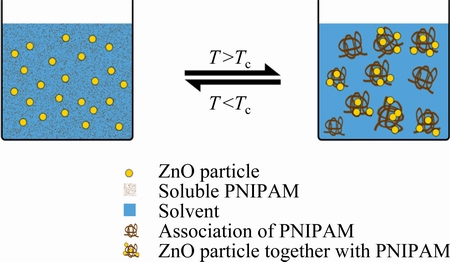
Fig. 5 Schematic diagram of gelation mechanism in colloidal processing with PNIPAM as coagulating agent
The existence of thermosensitive PNIPAM has a significant effect on the rheological behavior of ZnO suspension. In this suspension system, PAA as a kind of typical polyanion dispersant does not produce any chemical reaction with uncharged thermosensitive PNIPAM macromolecular. The schematic diagram of gelation mechanism in the colloidal processing using PNIPAM as a coagulating agent is shown in Fig. 5. It provides a reasonable explanation for this phenomenon. At a lower temperatures (T
In Fig. 3, the shear thinning behavior of ZnO suspension with high solid loading is more significant than that with low solid loading. This phenomenon is explained as that, in the ZnO suspension with high solid loading, the particles easily produce flocculation due to small average spacing between particles, the larger particle agglomerations in suspension are sheared into fine units as the shear rate increases. In addition, the ZnO suspension with the same solid loading at 40 °C is more remarkable than that at 20 °C. The precipitated tight particles of PNIPAM due to phase transformation at higher temperature enhance the effective volume fraction, which aggravates the agglomeration and flocculation between particles. This effect is the most severe at high particle loading. Therefore, the shear thinning behavior of 50% ZnO suspension with 8 mg/mL PNIPAM at 40 °C is the most apparent.
The viscosity of suspension is greatly affected by the concentration of PNIPAM and the solid loading [35]. In fact, both concentration and solid loading have contribution to the effective volume fraction. At a lower temperature, the polymers dissolving in water exist by stretching chains adsorbed on the surface of ZnO powder particles, which provides the adsorbed layer accounted for effective volume fraction (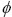 eff). The polymeric adsorbed layer thickness is estimated to be 4.24 nm for PAA with relative molecular mass of 5000 [36]. When the temperature increases, thermosensitive PNIPAM exhibits a phase transformation and forms solid spherical particles.
eff). The polymeric adsorbed layer thickness is estimated to be 4.24 nm for PAA with relative molecular mass of 5000 [36]. When the temperature increases, thermosensitive PNIPAM exhibits a phase transformation and forms solid spherical particles. 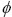 eff is enhanced by the volume occupied by the precipitated solid particles and the adsorbed layer around the ceramic particles. So, the effective volume fraction
eff is enhanced by the volume occupied by the precipitated solid particles and the adsorbed layer around the ceramic particles. So, the effective volume fraction 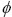 eff can be defined as
eff can be defined as
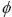 eff=
eff=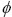 (1+δAsρs)+
(1+δAsρs)+ 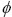 ′ (1)
′ (1)
where 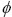 is solid loading of ZnO powder particles, δ is the thickness of adsorbed layer, As is the specific surface area of ceramic particles, ρs is the density of ceramic particles, and
is solid loading of ZnO powder particles, δ is the thickness of adsorbed layer, As is the specific surface area of ceramic particles, ρs is the density of ceramic particles, and 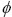 ′ is the solid loading of precipitated PNIPAM spherical particles at higher temperatures. According to Eq. (1), it can be concluded that the increase of PNIPAM concentration and solid loading can improve the effective volume fraction. Figure 6 shows the effect of effective volume fraction on the relative viscosity of ZnO suspension with 8 mg/mL PNIPAM. It can be seen that the relative viscosity of ZnO suspensions increases exponentially as the effective volume fraction increases. Obviously, the increase of solid loading reduces the average spacing among the powder particles, and thus the viscosity of ZnO suspension increases. The difference is that the growth rate of relative viscosity of suspension at 40 °C is higher. It can also be indentified from Fig. 6 that the growth index at 40 °C (n=4.6) is larger than that at 20 °C (n=3.6). The effective volume fraction consists of two parts at 40 °C, one is the volume fraction of ZnO powder particles and adsorbed layer as a whole, and the other is the volume fraction of solid spherical particles generated from the phase transition of thermosensitive PNIPAM. In contrast, the effective volume fraction at 20 °C only needs to consider the first part. Table 1 gives the
′ is the solid loading of precipitated PNIPAM spherical particles at higher temperatures. According to Eq. (1), it can be concluded that the increase of PNIPAM concentration and solid loading can improve the effective volume fraction. Figure 6 shows the effect of effective volume fraction on the relative viscosity of ZnO suspension with 8 mg/mL PNIPAM. It can be seen that the relative viscosity of ZnO suspensions increases exponentially as the effective volume fraction increases. Obviously, the increase of solid loading reduces the average spacing among the powder particles, and thus the viscosity of ZnO suspension increases. The difference is that the growth rate of relative viscosity of suspension at 40 °C is higher. It can also be indentified from Fig. 6 that the growth index at 40 °C (n=4.6) is larger than that at 20 °C (n=3.6). The effective volume fraction consists of two parts at 40 °C, one is the volume fraction of ZnO powder particles and adsorbed layer as a whole, and the other is the volume fraction of solid spherical particles generated from the phase transition of thermosensitive PNIPAM. In contrast, the effective volume fraction at 20 °C only needs to consider the first part. Table 1 gives the 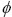 ′ of ZnO suspensions with 8 mg/mL PNIPAM at different solid loadings at higher temperature. The results indicate that although the volume fraction of solid spherical particles generated by precipitated PNIPAM is rather small in the order of 10-5, its contribution to the viscosity of ZnO suspension is enormous. In general, especially for the suspension with high solid loading, a small increase of solid loading can cause a dramatic increase in viscosity. The increase of effective volume fraction as result of phase transformation of thermosensitive PNIPAM at higher temperature is precisely the cause of increase in viscosity of ZnO suspension. It is indentified from Fig. 4 that, for the same reason, the storage modulus of suspension increases as the temperature rises. The colloidal suspension displays predominant viscosity at low temperature and shows a strongly elastic behavior at high temperatures because of the increase of effective volume fraction.
′ of ZnO suspensions with 8 mg/mL PNIPAM at different solid loadings at higher temperature. The results indicate that although the volume fraction of solid spherical particles generated by precipitated PNIPAM is rather small in the order of 10-5, its contribution to the viscosity of ZnO suspension is enormous. In general, especially for the suspension with high solid loading, a small increase of solid loading can cause a dramatic increase in viscosity. The increase of effective volume fraction as result of phase transformation of thermosensitive PNIPAM at higher temperature is precisely the cause of increase in viscosity of ZnO suspension. It is indentified from Fig. 4 that, for the same reason, the storage modulus of suspension increases as the temperature rises. The colloidal suspension displays predominant viscosity at low temperature and shows a strongly elastic behavior at high temperatures because of the increase of effective volume fraction.
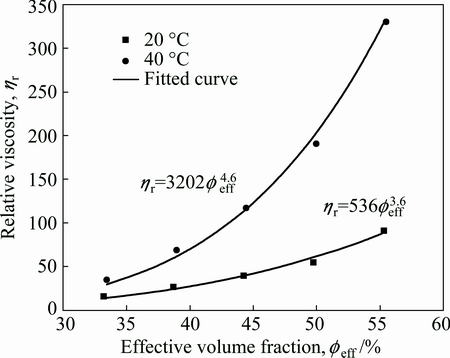
Fig. 6 Effect of effective volume fraction on relative viscosity of ZnO suspension with 8 mg/mL PNIPAM
Table 1 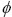 and
and 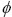 ′ of ZnO suspensions with 8 mg/mL PNIPAM at higher temperatures
′ of ZnO suspensions with 8 mg/mL PNIPAM at higher temperatures
For colloidal processing of ceramic, the largest possible solid loading is desired to reduce the constriction of ceramic body during drying and sintering process, and thus the maximum solid loading is especially important for suspension. As solid loading reaches the maximum value, the viscosity of suspension tends to infinity. There are many models used to calculate the maximum solid loading of ceramic suspension, such as Liu, Krieger-Dougherty, Mooney, Chong, and Dabak models [27,37,38]. Figure 7 illustrates the maximum solid loadings at 20 and 40 °C determined by linear fitting according to Liu and Chong models. In these models, the solid loadings are replaced with the effective solid loadings which reflect the actual volume fraction of suspensions. LIU [39] developed a model describing the relationship between solid loading and viscosity to predict the maximum solid loading of the suspension, as given by
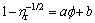 (2)
(2)
where ηr is the relative viscosity which is the ratio of suspension viscosity to solvent viscosity. Constants a and b are determined by linear fitting of Eq. (2). The value of maximum solid loading obtained by extrapolation is the solid loading when 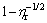 equals 1. Figure 7(a) indicates the curves of
equals 1. Figure 7(a) indicates the curves of 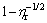 and effective volume fraction (feff) at 20 and 40 °C. In short, the viscosity of suspension increases as the solid loading increases. It is obvious that there is significant positive linear correlation between
and effective volume fraction (feff) at 20 and 40 °C. In short, the viscosity of suspension increases as the solid loading increases. It is obvious that there is significant positive linear correlation between 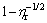 and effective volume fraction, and the corresponding value of the maximum solid loading (fm) is given at each temperature in this figure. The calculated values are in good agreement with the fitted results. It can be identified that the maximum solid loadings of the suspensions at 20 and 40 °C are 62.5% and 58.3%, respectively, which are defined as the values at which the viscosity of suspension tends to be infinity. However, Liu equation is suitable for ceramic suspensions with lower solid loadings. Then, the modified Chong equation which is suitable for suspensions with higher solid loading is defined as [40]:
and effective volume fraction, and the corresponding value of the maximum solid loading (fm) is given at each temperature in this figure. The calculated values are in good agreement with the fitted results. It can be identified that the maximum solid loadings of the suspensions at 20 and 40 °C are 62.5% and 58.3%, respectively, which are defined as the values at which the viscosity of suspension tends to be infinity. However, Liu equation is suitable for ceramic suspensions with lower solid loadings. Then, the modified Chong equation which is suitable for suspensions with higher solid loading is defined as [40]:
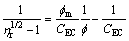 (3)
(3)
where CEC is a constant that is determined by linear fitting of Eq. (3). The maximum solid loading of suspensions is the solid loading when 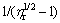 equals 0. Figure 7(b) gives the curves of
equals 0. Figure 7(b) gives the curves of 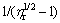 vs 1/feff at 20 and 40 °C. The maximum solid loadings of suspensions obtained by fitting Chong equation at 20 and 40 °C are 67.3% and 60.5%, respectively, and the simulation of the calculated values can coincide with the experimental ones. The results are slightly higher than those from Fig. 7(a), but the maximum solid loadings at 20 °C are higher than those at 40 °C in both figures. The phase transition of thermosensitive PNIPAM increases the effective volume fraction of ZnO suspension, thus reducing the dispersion stability of suspension and increasing the relative viscosity at high temperatures. Consequently, the maximum solid loading decreases with phase transition of PNIPAM. The fitted results in Fig. 7 also prove that the precipitation of PNIPAM has an important impact on the suspension.
vs 1/feff at 20 and 40 °C. The maximum solid loadings of suspensions obtained by fitting Chong equation at 20 and 40 °C are 67.3% and 60.5%, respectively, and the simulation of the calculated values can coincide with the experimental ones. The results are slightly higher than those from Fig. 7(a), but the maximum solid loadings at 20 °C are higher than those at 40 °C in both figures. The phase transition of thermosensitive PNIPAM increases the effective volume fraction of ZnO suspension, thus reducing the dispersion stability of suspension and increasing the relative viscosity at high temperatures. Consequently, the maximum solid loading decreases with phase transition of PNIPAM. The fitted results in Fig. 7 also prove that the precipitation of PNIPAM has an important impact on the suspension.
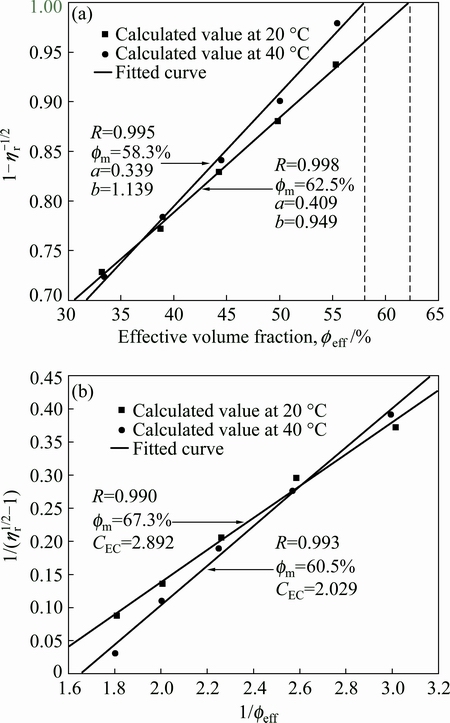
Fig. 7 Maximum solid loadings at 20 and 40 °C determined by linear fitting according to Liu equation (a) and Chong equation (b)
5 Conclusions
1) The low critical solution temperature (LCST) of PNIPAM aqueous solution is around 32 °C. PNIPAM exhibits a phase transition at LCST caused by the hydrophobic association between polymer chains and the damage of hydrogen bond between polymer and water upon heating.
2) The viscosity of ZnO suspension with PNIPAM increases sharply when the temperature is higher than 40 °C. Two main reasons are the increment of effective volume fraction stemming from physical association between the PNIPAM segments and the flocculation of organic associations of PNIPAM and inorganic powder particles. When the temperature is higher than 40 °C, the viscosity of 50% ZnO suspension with 8 mg/mL PNIPAM increases rapidly and reaches a high value of 11.49 Pa·s at 50 °C, and the colloidal suspension changes from predominately viscous to strongly elastic.
3) The rheological behavior is related to the concentration of PNIPAM and solid loading of suspension, both of them can contribute to the effective volume fraction. The volume fraction generated by precipitated PNIPAM particles is rather small on the order of 10-5, but its contribution to the viscosity of ZnO suspension is enormous.
4) The maximum solid loadings obtained by fitting the experimental results are 62.5% at 20 °C and 58.3% at 40 °C according to Liu equation, and 67.3% at 20 °C and 60.5% at 40 °C according to Chong equation. The results indicate that the precipitation of PNIPAM has a significant influence on the rheological behavior of ZnO suspension.
References
[1] LIN B, DONG Y, WANG S, FANG D, DING H, ZHANG X, LIU X, MENG G. Stable, easily sintered BaCe0.5Zr0.3Y0.16Zn0.04O3-d electrolytebased proton-conducting solid oxide fuel cells by gel-casting and suspension spray [J]. Journal of Alloys and Compounds, 2009, 478(1): 590-593.
[2] WANG X F, WANG R C, PENG C Q, LI H P, LIU B, WANG Z Y. Thermoresponsive gelcasting: Improved drying of gelcast bodies [J]. Journal of the American Ceramic Society, 2011, 94(6): 1679-1682.
[3] WANG Xiao-feng, WANG Ri-chu, PENG Chao-qun, LI Ting-ting, LUO Yu-lin, WANG Chao, LIU Bing. Research and development of gelcasting [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(3): 496-509. (in Chinese)
[4] SUZUKI T S, UCHIKOSHI T, SAKKA Y. Effect of sintering conditions on microstructure orientation in α-SiC prepared by slip casting in a strong magnetic field [J]. Journal of the European Ceramic Society, 2010, 30(14): 2813-2817.
[5]  S, ANEZIRIS C G. Pressure slip casting of coarse grain oxide ceramics [J]. Ceramics International, 2012, 38(1): 417-422.
S, ANEZIRIS C G. Pressure slip casting of coarse grain oxide ceramics [J]. Ceramics International, 2012, 38(1): 417-422.
[6] SUN Yi-hua, XIONG Wei-hao, LI Chen-hui. Fabrication of ultrahigh density ZnO-Al2O3 ceramic composites by slip casting [J]. Transaction of Nonferrous Metals Society of China, 2010, 20(4): 624-631.
[7] LEWIS J A, EMAY J E, STUECKER J, CESARANO III J. Direct ink writing of three-dimensional ceramic structures [J]. Journal of the American Ceramic Society, 2006, 89(12): 3599-3609.
[8] SUN Yue-hua, PENG Chao-qun, WANG Xiao-feng, WANG Ri-chu, CHEN Yi-xin. Direct ink writing: A novel avenue for engineering micro-/nanoscale 3D structures [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(6): 1525-1537. (in Chinese)
[9] ZHOU Zhen-jun, YANG Zheng-fang, YUAN Qi-ming. Barium titanate ceramic inks for continuous ink-jet printing synthesized by mechanical mixing and sol-gel methods [J]. Transaction of Nonferrous Metals Society of China, 2008, 18(1): 150-154.
[10] BALZER B, HRUSCHKA M K M, GAUCKLER L J. Coagulation kinetics and mechanical behavior of wet alumina geen bodies produced via DCC [J]. Journal of Colloid and Interface Science, 1999, 216(2): 379-386.
[11] TERVOORT E, TERVOORT T A, GAUCKLER L J. Chemical aspects of direct coagulation casting of alumina suspensions [J]. Journal of the American Ceramic Society, 2004, 87(8): 1530-1535.
[12] WANG Xiao-feng, SUN Yue-hua, PENG Chao-qun, WANG Ri-chu, ZHANG Dou. Research and development of direct coagulation casting [J]. The Chinese Journal of Nonferrous Metals, 2015, 25(2): 267-279. (in Chinese)
[13] XU J, QU Y N, XI X Q, YANG J L. Properties of alumina coagulated bodies prepared by direct coagulation casting via high valence counter ions (DCC-HVCI) [J]. Journal of the American Ceramic Society, 2012, 95(11): 3415-3420.
[14] XU J, WEN N, LI H X, QI F, XI X Q, YANG J L. Direct coagulation casting of alumina suspension by high valence counter ions using Ca(IO3)2 as coagulation agent [J]. Journal of the American Ceramic Society, 2012, 95(8): 2525-2530.
[15] BALZER B, HRUSCHKA M K M, GAUCKLER L J. In situ rheological investigation of the coagulation in aqueous alumina suspensions [J]. Journal of the American Ceramic Society, 2001, 84(8): 1733-1739.
[16] LI W, ZHANG H X, JIN Y P, GU M Y. Rapid coagulation of silicon carbide slurry via direct coagulation casting [J]. Ceramics International, 2004, 30(3): 411-416.
[17] PRABHAKARAN K, MELKERI A, GOKHALE N M, CHONGDAR T K, SHARMA S C. Direct coagulation casting of YSZ powder suspensions using MgO as coagulating agent [J]. Ceramics International, 2009, 35(4): 1487-1492.
[18] PRABHAKARAN K, JOSEPH K, SOORAJ R, DURGAPRASAD C. Magnesia induced coagulation of aqueous PZT powder suspensions for direct coagulation casting [J]. Ceramics International, 2010, 36(7): 2095-2101.
[19] YANG J L, XU J, WEN N, QU Y N, QI F, XI X Q. Direct coagulation casting of alumina suspension via controlled release of high valence counterions from thermo-sensitive liposomes [J]. Journal of the American Ceramic Society, 2013, 96(1): 62-67.
[20] XU J, ZHANG Y X, QU Y N, QI F, ZHANG X Y, YANG J L. Direct coagulation casting of alumina suspension from calcium citrate assisted by pH shift [J]. Journal of the American Ceramic Society, 2014, 97(4): 1048-1053.
[21] WANG X F, SUN Y H, PENG C Q, ZHANG D, CHEN Y X, WANG R C. Colloidal processing of ZnO using thermosensitive poly(N-isopropylacrylamide) as a coagulating agent [J]. Ceramics International, 2015, 41(7): 9163-9167.
[22] WANG X F, LIU P, TIAN Y. Preparation and drug release behavior of temperature-responsive mesoporous carbons [J]. Journal of Solid State Chemistry, 2011, 184(6): 1571-1575.
[23] XIA Y, YIN X, BURKE N A D,  H D H. Thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization [J]. Macromolecules, 2005, 38(14): 5937-5943.
H D H. Thermal response of narrow-disperse poly(N-isopropylacrylamide) prepared by atom transfer radical polymerization [J]. Macromolecules, 2005, 38(14): 5937-5943.
[24] WU C, ZHOU S. Volume phase transition of swollen gels: Discontinuous or continuous [J]. Macromolecules, 1997, 30(3): 574-576.
[25] PAMIES R, ZHU K,  Thermal response of low molecular weight poly(N-isopropylacrylamide) polymers in aqueous solution [J]. Polymer Bulletin, 2009, 62(4): 487-502.
Thermal response of low molecular weight poly(N-isopropylacrylamide) polymers in aqueous solution [J]. Polymer Bulletin, 2009, 62(4): 487-502.
[26] LEWIS J A. Colloidal processing of ceramics [J]. Journal of the American Ceramic Society, 2000, 83(10): 2341-2359.
[27] SONG Y L, LIU X L, CHEN J F. The maximum solid loading and viscosity estimation of ultra-fine BaTiO3 aqueous suspensions [J]. Colloids and Surfaces A (Physicochemical and Engineering Aspects), 2004, 247(1-3): 27-34.
[28] NAHAIN A A, LEE K S, MOSAIAB T, PARK S Y. pH and thermo-responsive poly(N-isopropylacrylamide) copolymer grafted to poly(ethylene glycol) [J]. Journal of Applied Polymer Science, 2013, 130(1): 168-174.
[29] LIN C L, CHIU W Y, LEE C F. Preparation, morphology, and thermoresponsive properties of poly(N-isopropylacrylamide)-based copolymer microgels [J]. Journal of Polymer Science Part A: Polymer Chemistry, 2006, 44(1): 356-370.
[30]  Temperature induced gelation of concentrated ceramic suspensions: rheological properties [J]. Journal of the European Ceramic Society, 1999, 19(12): 2117-2123.
Temperature induced gelation of concentrated ceramic suspensions: rheological properties [J]. Journal of the European Ceramic Society, 1999, 19(12): 2117-2123.
[31] XU X, FERREIRA J M F. Temperature-induced gelation of concentrated sialon suspensions [J]. Journal of the American Ceramic Society, 2005, 88(3): 593-598.
[32] YANG Y, SIGMUND W M. A new approach to prepare highly loaded aqueous alumina suspensions with temperature sensitive rheological properties [J]. Journal of the European Ceramic Society, 2003, 23(2): 253-261.
[33] EWAIS E, ZAMAN A A, SIGMUND W. Temperature induced forming of zirconia form aqueous slurries: Mechanism and rheology [J]. Journal of the European Ceramic Society, 2002, 22(16): 2805-2812.
[34] ZHANG Y, UEMATSU K. A novel consolidation approach for ceramic colloidal forming [C]//Proceedings of CICC-1: The First China International Conference on High-Performance Ceramics. Beijing, 1999.
[35] ERENCIA M, MESAS M L, NAVARRETE F C. Thermosensitive fibres of lyocell/poly(N-isopropylacrylamide): Multiparametric analysis for studying the graft copolymerization [J]. Polymer International, 2013, 62(9): 1316-1323.
[36] NAPPER D H. Polymeric stabilization of colloidal dispersions [M]. New York: Academic Press, 1982.
[37] WANG X F, WANG R C, PENG C Q, LI H P. Rheology of aqueous BeO suspension with NH4PAA as a diapersant [J]. Progress in Natural Science: Materials International, 2012, 22(4): 347-353.
[38] BINNER J G P, MCDERMOTT A M. Rheological characterisation of ammonium polyacrylate dispersed, concentrated alumina suspensions [J]. Ceramics International, 2006, 32(7): 803-810.
[39] LIU D M. Particle packing and rheological property of highly- concentrated ceramic suspensions: 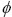 m determination and viscosity prediction [J]. Journal of Materials Science, 2000, 35(21): 5503--5507.
m determination and viscosity prediction [J]. Journal of Materials Science, 2000, 35(21): 5503--5507.
[40] EDIRISINGHE M J, SHAW H M, TOMKINS K L. Flow behavior of ceramic injection moulding suspensions [J]. Ceramics International, 1992, 18(3): 193-200.
孙月花,彭超群,王小锋,王日初,冯 艳,王乃光
中南大学 材料科学与工程学院,长沙 410083
摘 要:采用温敏性聚(N-异丙基丙烯酰胺)(PNIPAM)为凝固剂的新型胶态成型已应用于制备复杂形状的陶瓷器件。本文作者研究PNIPAM水溶液性能和添加PNIPAM的ZnO悬浮液的流变性能。结果表明,PNIPAM表现出明显的温敏性且转变温度在32 °C左右。当温度高于40 °C (Tc,温敏悬浮液的临界转变温度),添加8 mg/mL PNIPAM的50% ZnO(体积分数)悬浮液的黏度急剧增加,温度为50 °C时黏度高达11.49 Pa·s并表现出较强弹性。原因在于高温下PNIPAM分子的析出导致悬浮液的有效固相体积分数增加以及ZnO陶瓷颗粒之间的絮凝作用。此外,20 °C下悬浮液的最大固相体积分数明显高于40 °C下的,也可证明PNIPAM的相转变可诱导悬浮液的絮凝。
关键词:流变行为;ZnO;聚(N-异丙基丙烯酰胺);温敏性;有效固相体积分数
(Edited by Wei-ping CHEN)
Foundation item: Project (51202296) supported by the National Natural Science Foundation of China; Project (20120162120006) supported by the Specialized Research Fund for the Doctoral Program of Higher Education, China
Corresponding author: Xiao-feng WANG; Tel/Fax: +86-731-88836638; E-mail: 13467516329@163.com
DOI: 10.1016/S1003-6326(16)64423-0

