文章编号:1004-0609(2015)-05-1294-06
共沉淀-氢气还原法制备碲化镉粉末
刘 远,郑雅杰,孙召明
(中南大学 冶金与环境学院,长沙 410083)
摘 要:以高纯CdCl2、TeCl4和氨水为原料,采用共沉淀-氢气还原法制备出碲化镉粉末。从离子反应平衡角度,对碲和镉共沉淀产物的制备过程进行热力学计算,得到Cd2+-Te4+- NH3-H2O体系金属离子浓度与pH值之间的关系,可以得到在7.0<pH<8.0时,体系处于碲和镉的共沉淀区域。结果表明:在Cd2+、Te4+浓度在0.1mol/L的溶液中,加入氨水调节终点pH=7.1时,碲和镉的共沉淀率为99.5%,此时碲镉共沉淀产物为Cd(OH)2、TeO2和少量Cd(NH3)2Cl2的混合物。然后采用氢气还原碲和镉共沉淀产物,在反应温度为400 ℃、保温时间为2.5 h、氢气流量为40 L/h时,成功制备碲化镉粉末。
关键词:碲化镉粉末;共沉淀;氢气还原
中图分类号:TF114.1 文献标志码:A
CdTe powder prepared by coprecipitation-hydrogen reduction method
LIU Yuan, ZHENG Ya-jie, SUN Zhao-ming
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: CdTe powder was prepared by the coprecipitation-hydrogen reduction method using high purity CdCl2, TeCl4 and ammonia as the raw materials. The preparation process of the Cd and Te coprecipitation product was analyzed by thermodynamic calculation, and the relationship between metal ion concentration and pH at 25 ℃ for Cd2+-Te4+-NH3-H2O system was established. The system is in the co-precipitation area of Cd and Te when 7.0<pH<8.0. The results show that the coprecipitation rate of is 99.5% as the concentrations of Cd and Te are 0.1 mol/L when pH=7.1. XRD results show that the coprecipitation product is a mixture of Cd(OH)2, TeO2 and small amount of Cd(NH3)2Cl2. The Cd and Te coprecipitation product is reduced by H2. As the reaction temperature is 400 ℃, isothermal time is 2.5 h, hydrogen flow is 40 L/h, the CdTe powder can be successfully synthesized.
Key words: CdTe powder; coprecipitation; hydrogen reduction
碲化镉作为一种重要的Ⅱ-Ⅵ族半导体材料[1],由于其独特的光学、电学特性,在发光二级管[2]、非线性光学[3]、太阳能电池和生命科学等领域获得广泛应用。现在采用湿化学法[4]合成碲化镉粉末的制备技术发展很快,具有代表性的方法有微波辐射法[5]、微乳液法[6]、溶胶凝胶法[7-9],水热合成法[10-11],共沉淀法[12-13]以及相对传统的高温固相法[14-15]等。湿化学法的特点是在溶液中将所有组分均匀混合,然后共同分离,因此,湿化学法是制备具有精确化学计量比的复合粉体的优越方法。研究结果表明,湿化学法共沉淀制备的难点在于如何将多种元素按照化学计量比的共同沉淀,而Cd2+与NH3·H 2O作用时,会生成多种镉氨配离子,对共沉淀过程有一定影响[16]。本文作者从离子反应平衡角度,考虑溶液中镉的氨基配合离子,对Te-Cd-NH3-H2O体系进行热力学分析,研究体系中各溶解组分的平衡浓度随pH值的变化趋势,绘制体系的优势区域图,得到碲镉按照化学计量比的共沉淀区,在共沉淀区制备得到符合化学计量比的碲镉共沉淀产物。在前期采用氢气还原Cd(OH)2制得镉粉的研究基础上[17],本文作者以高纯CdCl2、TeCl4和氨水为原料,采用氢气还原碲镉共沉淀产物,制备出具有精确化学计量比的碲化镉粉末。
1 实验
1.1 实验原料
实验中使用的高纯Cd和Te原料为实验室制备[17-18],其杂质含量分别如表1和2所列。使用的氨水为分析纯,使用的氢气纯度为99.99%。
表1 原料CdCl2的杂质含量
Table 1 Impurities contents in raw material CdCl2 (mg/kg)

表2 原料TeCl4的杂质含量
Table 2 Impurities contents in raw material TeCl4 (mg/kg)

1.2 实验步骤
按照n(Cd)/n(Te)=1:1,取CdCl2溶液和TeCl4溶液配制成混合溶液,加入氨水调节溶液pH值为7.0,得到碲镉共沉淀物,将其洗涤干燥后置于程控管式炉中,在一定的温度下氢气气氛中还原得到碲化镉粉体。
1.3 分析与检测
采用电感耦合等离子光谱仪(Intrepid II XPS)分析物料中杂质含量;采用Rint-2000型X射线衍射仪分析样品的物相组成;采用扫描电镜(JEOL,JSM-5600LV)对样品的形貌进行表征。
2 结果与讨论
2.1 Cd2+-Te4+- NH3-H2O体系中金属离子浓度与pH值的关系
在Cd2+-Te4+- NH3-H2O复杂体系中,Cd(II)与NH3形成多种配合物,该体系中不存在氧化还原反应,根据同时平衡原理,每种镉配离子均与Cd(OH)2平衡,计算中采用浓度代替活度。在Cd2+和Te4+水溶液中,加入氨水时,发生的沉淀反应如式(1)和(2)所示:
Te4++4OH-=Te(OH)4↓ (Ksp1=3×10-54) (1)
Cd2++2OH-=Cd(OH)2↓ (Ksp2=7.2×10-15) (2)
由式(1)和(2)可以看出,在体系中Te4+先发生沉淀,当c(Cd2+)·c(OH-)2>7.2×10-15时,Cd(OH)2才与Te(OH)4共同沉淀。由式(1)和(2),根据平衡反应得
c(Te4+)·c(OH-)4=3×10-54 (3)
lgc(Te4+)=-4pH+2.48 (4)
同理可得
lgc(Cd2+)=-2pH+12.8 (5)
当满足lgc(Cd2+)≥12.8-2pH和lgc(Te4+)≥2.48-4pH时,混合体系才能发生共沉淀。在此体系中要考虑到Cd2+与氨发生络合反应,生成镉氨络合物,对平衡体系有一定的影响,会影响共沉淀产物中碲和镉的配比。25 ℃时,体系中Cd2+和NH3的有效平衡反应及其ΔrGmΘ如式(6)所示[19]:
Cd(OH)2+2NH3=Cd(NH3)22++2OH-(ΔrGmΘ=130.99 kJ/mol) (6)
式(6)中有
ΔrGm=ΔrGmΘ+RTlnc[Cd(NH3)2]2++2RTlnc(OH-) (7)
得
lnc[Cd(NH3)2]2+=-2pH+14.8 (8)
Cd(OH)2+4NH3=Cd(NH3)42++4OH-(ΔrGmΘ=39.32 kJ/mol) (9)
式(9)中有
ΔrGm=ΔrGmΘ+RTlnc[Cd(NH3)4]2++4RTlnc(OH-) (10)
得
lnc[Cd(NH3)4]2+=-2pH+18.15 (11)
根据式(4)、(5)、(8)和(9)可知,在Cd2+-Te4+-NH3- H2O体系中金属离子浓度及pH值的关系(见图1)。图1中每条直线表示与Te(OH)4和Cd(OH)2固相平衡时对应的离子浓度与pH值关系。为了保证共沉淀得到具有精确化学计量比的碲镉混合物,应控制反应在图1中区域Ⅲ进行,即碲镉共沉淀区域。
2.2 氨水沉淀法制备碲镉共沉淀物
采用CdCl2溶液和TeCl4溶液配制Cd2+和Te4+浓度各为0.1mol/L的混合溶液,在搅拌作用下加入氨水溶液,采用PHS-3C数字酸度计测定反应过程的pH值,反应结束后将共沉淀物直接置于60℃真空干燥箱中烘干得到碲镉混合共沉淀。图2所示为不同终点pH值下的碲镉共沉淀率。由图2可知,在溶液中n(Cd2+)=n(Te4+)=0.1 mol/L时,随着pH值的增加,碲和镉共沉淀率逐渐增大,当pH=7.1时,碲镉共沉淀率为99.5%。随着pH值继续增加,镉的沉淀率开始下降,pH=8时,镉沉淀率降低至95.4%。结合图1可知,当n(Cd2+)=n(Te4+)=0.1 mol/L,pH=8时,镉会生成Cd(NH3)22+溶解于溶液中,造成镉沉淀率降低,影响共沉淀物中碲镉的配比,因此,在此共沉淀过程中应控制反应终点pH=7.0。
图3所示为终点pH=7.1时所得碲镉共沉淀物的XRD谱。由图3可知,由于在烘干过程中Te(OH)4分解,碲镉共沉淀产物中碲主要以TeO2形式存在,镉以Cd(OH)2和Cd(NH3)2Cl2形式存在。Cd(NH3)2Cl2的生成说明在NH3·H2O体系中少量的Cd(OH)2生成[Cd(NH3)2]2+配合离子,从而产生Cd(NH3)2Cl2,这与前面的热力学分析结果是一致的,当碲镉共沉淀产物直接过滤烘干,Cd(NH3)2Cl2残存在其中。

图1 Cd2+-Te4+- NH3-H2O体系的lgc-pH图
Fig. 1 Relationship between lgc and pH of Cd2+-Te4+- NH3-H2O system

图2 不同pH值下碲和镉的共沉淀率
Fig. 2 Coprecipitation rates of Te and Cd at different final pH values
图4所示为共沉淀制备的碲镉共沉淀的SEM像。从图4中可以看出,共沉淀得到的粉末粒度分布比较均匀,颗粒疏松多孔,超大团聚颗粒少。

图3 pH=7.1时共沉淀产物的XRD谱
Fig. 3 XRD pattern of coprecipitated product at final pH value of 7.1
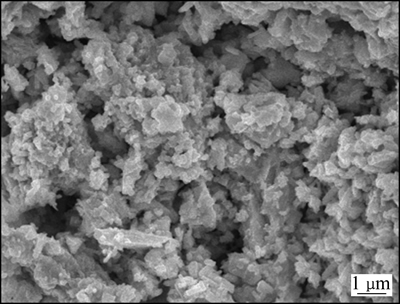
图4 碲镉共沉淀的SEM像
Fig. 4 SEM image of Te-Cd coprecipitated product
2.3 氢气还原碲镉共沉淀产物制备碲化镉粉末
将烘干后的碲镉共沉淀产物研磨后,放入石英舟内并置于管式炉中,通入氮气置换管内空气后通入氢气,升温至指定温度,还原时间为2.5 h。图5所示为不同还原温度下产物的XRD谱。由图5可知,采用氢气还原碲镉共沉淀产物,当还原温度为250~300 ℃时,还原产物中存在TeO2和[Cd(NH3)2]Cl2的物相;当反应温度高于400 ℃时,TeO2和[Cd(NH3)2]Cl2物相消失,还原产物为纯净的CdTe;继续升高还原温度至450 ℃,产物的衍射峰尖锐,产物为立方闪锌矿结构CdTe。
图6所示为在不同反应温度下产物的SEM像。从图6(a)可以看出,当还原温度为400 ℃时,产品表面及内部存在大量孔隙且分布较均匀。从图6(b)中可以看出,当还原温度为450 ℃时,产物表面生成致密层。因此,反应温度对产物形貌的影响很大。在可合成单一相CdTe的条件下,温度为400 ℃时CdTe粒度较小,表面疏松;当温度增大至450 ℃,CdTe粒度增大,表面变光滑致密。

图5 在不同还原温度下产物的XRD谱
Fig. 5 XRD patterns of products prepared at various reduction temperatures

图6 不同还原温度下产物的SEM像
Fig. 6 SEM images of products at different reduction temperatures
在氢气还原碲镉共沉淀产物的过程中,可能发生化学反应如式(12)~(16)所示:
CdO+H2=Cd+H2O (12)
TeO2+2H2=Te+2H2O (13)
Cd+Te=CdTe (14)
[Cd(NH3)2]Cl2=CdCl2+2NH3 (15)
CdCl2+TeO2+H2=CdTe+H2O (16)
反应中物质在相应温度下的吉布斯生成自由能△GΘ如表3所列[15]。
表3 体系部分成分在不同温度下的△GΘ[15]
Table 3 △GΘ of some compositions in this system at different temperatures

通过热力学计算得到式(12)~(16)的△GΘ与T的关系,可以看出当反应温度高于600 K时,均有△GΘ<0,即在600 K以上式(12)~(16)都可能进行。共沉淀过程中产生的[Cd(NH3)2]Cl2分解后与TeO2在氢气的作用下还原生成CdTe,挥发的水蒸气在反应表面形成许多孔洞,有利于充分还原得到CdTe。
图7所示为在还原温度为400 ℃、还原时间为1、2.5、3.5和4.5 h时所得产物的XRD谱。由图7可知,在还原时间为1 h时,所得产物显示出明显的CdCl2·H2O特征峰,说明在还原反应初始阶段发生了式(15),在2θ为23.796°、39.351°、46.448°、62.457°等处出现CdTe特征峰,说明此时已经初步生成CdTe晶体,但峰强不高,还处于无定形状态。从图7中还可以看出,随着还原时间的增加,CdCl2·H2O特征峰消失,CdTe衍射峰强度明显增大,说明晶型结构趋于完整,在还原2.5 h时,除了立方闪锌矿结构的CdTe相特征峰外,未发现其他杂相的衍射峰,产物相成分均一。
图8所示为在还原温度为400 ℃、不同还原时间时所得产物的SEM像。从图8中可以看出,随着还原时间的升高,晶体颗粒逐渐长大,在还原时间达4.5 h时,颗粒分布不均匀,形状变得不规则,颗粒团聚成块。这是由于随着还原时间的延长,小颗粒溶解再结晶,与其他颗粒团聚于一体,粒径增大。在一定的还原温度下,晶粒的平均直径与时间的平方根成正比,产物的平均尺寸随着反应时间的增加而增加,由晶粒聚集的颗粒尺寸也增大。随着还原时间的增加,细颗粒发生团聚,粗粒区的颗粒逐渐增加,碲化镉的粒径明显变大。因此,在保证产品质量的同时应尽量减少反应时间,从而控制碲化镉粉体粒径的增大。

图7 不同还原时间下所得产物的XRD谱
Fig. 7 XRD patterns of products prepared for different reduction time

图8 不同还原时间下产物的SEM像
Fig. 8 SEM images of products prepared at different reduction time
对还原温度为400 ℃、还原时间为2.5 h时得到碲化镉粉体进行ICP分析,表4所列为3个单独样品的成分。由表4可知,碲和镉的质量分数符合CdTe的化学计量比。
表5所列为得到碲化镉粉末中的杂质含量。由表5可知,还原得到的碲化镉纯度达到99.99%。
表4 产物中Cd元素和Te元素的质量分数
Table 4 Mass fractions of Cd and Te in product

表5 还原产物CdTe中的杂质含量
Table 5 Impurities contents in CdTe powder (mg/kg)

3 结论
1) 根据热力学计算结果,得到25 ℃时Cd2+-Te4+- NH3-H2O体系的lgc-pH图。通过热力学分析可知,当溶液中n(Cd2+)=n(Te4+)=0.1 mol/L,当7<pH<8时,体系处于碲镉共沉淀区域。
2) 以氯化镉、氯化碲和氨水为原料,在水溶液中控制反应终点pH=7.1,制备了具有精确化学计量比的碲镉共沉淀产物。将此共沉淀产物置于氢气气氛下,在400 ℃下还原2.5 h成功制备了立方闪锌矿结构CdTe粉末,该粉末具有多孔结构,其纯度达到99.99%。
REFERENCES
[1] ANTONIO L, STEVEN H. Handbook of photovoltaic science and engineering[M]. United Kingdon: John Wiley & Sons Inc, 2011: 617-662.
[2] RABBANI M M, NAM D, KIM D, OH W. Characterization of Au/CdTe nanocomposites prepared by electrostatic interaction[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(2): 426-432.
[3] PAUDEL N R, WIELAND K A, COMPAAN A D. Ultrathin CdS/CdTe solar cells by sputtering[J]. Solar Energy Materials and Solar Cells, 2012, 105: 109-112.
[4] 刘雪岩, 杨丽君, 金燕利, 张 蕾, 徐天赐, 李 娜. 纳米TiO2对镉(II)的吸附性能[J]. 中国有色金属学报, 2011, 21(11): 2971-2977.
LIU Xue-yan, YANG Li-jun, JIN Yan-li, ZHANG Lei, XU Tian-ci, LI Na. Adsorption properties of nano-TiO2 for Cd(II)[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(11): 2971-2977.
[5] 尤晓刚, 贺 蓉, 高 峰, 邵 君, 潘碧峰, 崔大祥. 核/壳型 CdTe@SiO2荧光纳米复合粒子的制备与表征[J]. 化学学报, 2007, 65(4): 561-565.
YOU Xiao-gang, HE Rong, GAO Feng, SHAO Jun, PAN Bi-feng, CUI Da-he. Preparation and characterization of CdTe@ SiO2 core/shell luminescent composite nanoparticles[J]. Acta Chimica Sinica, 2007, 65(4): 561-565.
[6] YANG Hai-hua, FAN Wen-guang, ALEKSANDAR V, ANDREI S S, WEY Y T, ANDREY L R. Heterojunction engineering of CdTe and CdSe quantum dots on TiO2 nanotube arrays: Intricate effects of size-dependency and interfacial contact on photoconversion efficiencies[J]. Advanced Functional Materials, 2012, 22(13): 2821-2829.
[7] ABDOLAHZADEH Z A, GHODSI F E. Growth, characterization and studying of sol-gel derived CdS nanoscrystalline thin films incorporated in polyethyleneglycol: Effects of post-heat treatment[J]. Solar Energy Materials and Solar Cells, 2012, 105: 249-262.
[8] REISFELD R. Nanosized semiconductor particles in glasses prepared by the sol-gel method: Their optical properties and potential uses [J]. Journal of Alloys and Compounds, 2002, 341(1/2): 56-61.
[9] LIU Ning, YANG Ping. Highly stable functional sol-gel CdTe-SiO2 films with tunable emission[J]. Nanoscience and Nanotechnology Letters, 2012, 4(4): 384-388.
[10] YANG Wei-hai, LI Wan-wan, DOU Hong-jing, SUN Kang. Hydrothermal synthesis for high-quality CdTe quantum dots capped by cysteamine[J]. Materials Letters, 2008, 62(17/18): 2564-2566.
[11] WANG Jing, HAN He-you. Hydrothermal synthesis of high-quality type-II CdTe/CdSe quantum dots with near-infrared fluorescence[J]. Journal of Colloid and Interface Science, 2010, 351(1): 83-87.
[12] YU H F, ZHONG W B. Ultrafine MnFe2O4 powder preparation by combusting the coprecipitate with and without Mg2+ or Zn2+ additives[J]. Journal of Materials Research, 2000, 15(1): 170-175.
[13] BAHMANI A, SELLAMI M, BETTAHAR N. Synthesis of bismuth mixed oxide by thermal decomposition of a coprecipitate precursor[J]. Journal of Thermal Analysis and Calorimetry, 2012, 107(3): 955-962.
[14] LEE J H, LIM D G, YI J S. Electrical and optical properties of CdTe films prepared by vacuum evaporation with close spacing between source and substrate [J]. Solar Energy Materials and Solar Cells, 2003, 75(1): 235-242.
[15] RUSU M, NICOLAESCU I I, RUSU G G. Influence of deposition conditions on the structural characteristics of sublimated CdTe thin films[J]. Applied Physics A, 2000, 70(5): 565-571.
[16] TASKER P A, PLIEGER P G, WEST L C. Metal complexes for hydrometallurgy and extraction[J]. Comprehensive Coordination Chemistry Ⅱ, 2004, 9: 759-808.
[17] 刘 远, 郑雅杰, 孙召明. 锌冶炼含镉烟尘制备高纯镉粉的新工艺[J]. 中国有色金属学报,2014, 24(4): 1070-1075.
LIU Yuan, ZHENG Ya-jie, SUN Zhao-ming. New technology of high purity Cd powder prepared from roasting dust of zinc smelting[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(4): 1070-1075.
[18] LIU Yuan, ZHENG Ya-jie, SUN Zhao-ming. Preparation of high-purity tellurium powder by hydrometallurgical method[J]. Rare Metals, 2014, 33(4): 479-484.
[19] SPEIGHT J G. Lange’s handbook of chemistry[M]. New York: McGraw-Hill Companies, 2005: 1267-1289.
(编辑 王 超)
基金项目:广东省科技厅资助项目(2011B0508000033)
收稿日期:2014-08-11;修订日期:2015-01-20
通信作者:郑雅杰,教授,博士;电话:0731-88836285;E-mail:zyj@csu.edu.cn