
J. Cent. South Univ. (2020) 27: 27-36
DOI: https://doi.org/10.1007/s11771-020-4275-4
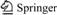
Kinetics of leaching lithium from lepidolite using mixture of hydrofluoric and sulfuric acid
WANG Hai-dong(王海东)1, ZHOU An-an(周安安)1, GUO Hui(郭慧)1, 2,LU Meng-hua(吕梦华)1, YU Hai-zhao(余海钊)1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: The fluorine-based chemical method shows great potential in leaching lithium (Li) from lepidolite. Leaching kinetics of Li in a mixture of sulfuric acid and hydrofluoric acid, which is a typical lixivant for the fluorine-based chemical method, was carried out under crucial factors such as different HF/ore ratios (1:1-3:1 g/mL) and leaching temperatures (50-85 °C). The kinetics data fit well with the developed shrinking-core model, indicating that the leaching rate of Li was controlled by the chemical reaction and inner diffusion at the beginning of leaching (0-30 min) as a calculated apparent activation energy (Ea) of 20.62 kJ/mol. The inner diffusion became the rate-limiting step as the leaching continues (60-180 min). Moreover, effects of HF/ore ratio and leaching temperature on selective leaching behavior of Li, Al and Si were discussed. 90% of fluorine mainly existed as HF/F- in leaching solution, which can provide theoretical guidance for further removal or recovery of F.
Key words: lepidolite; lithium extraction; fluorine-based chemical method; hydrofluoric acid; selective leaching; leaching kinetics
Cite this article as: WANG Hai-dong, ZHOU An-an, GUO Hui, LU Meng-hua, YU Hai-zhao. Kinetics of leaching lithium from lepidolite using mixture of hydrofluoric and sulfuric acid [J]. Journal of Central South University, 2020, 27(1): 27-36. DOI: https://doi.org/10.1007/s11771-020-4275-4.
1 Introduction
Lithium (Li) is projected to experience an increasing demand in the coming years due to its incredible application of lithium on primary and secondary batteries in electrical vehicles, portable devices and other energy storage materials. Currently, lithium is mainly extracted from lithium brines. However, about 70% of the global lithium brines are distributed in Argentina, Bolivia and Chile [1-4]. To diversify the lithium resource, the Li-bearing minerals, including spodumene, zinnwaldite and lepidolite have received increasing attention as important alternative Li resources due to its relatively wide distributions and shorter production cycle [4-10]. Different methods have been proposed aiming to extract lithium from lepidolite and listed in Table 1. The sulfuric acid method has been currently considered as the most efficient processes. However, the low lithium grade (~3% Li2O in concentrates) resulted in a much more complex process to economically extract Li than that from spodumene, which has been realized commercial production. Moreover, the lepidolite sampled from Yichun, Jiangxi Province, China is obtained as the flotation tailings of Ta and Nb. Therefore, more efficient methods need to be proposed to enhance Li leaching from lepidolite.
Here, a fluorine-based chemical method has been proposed considering that 2%-4% fluorine (F) contained in lepidolite. The dissolution reaction of lepidolite in hydrofluoric acid and sulfuric acid (HF/H2SO4) occurred as reactions 1-3 [8, 11, 12].
Table 1 Different methods to extract lithium from lepidolite
More detailed discussion on dissolution behavior has been presented in our previous work [8]. More importantly, the added H+ can influence the complex equilibrium between Al3+ and F- as reaction 2 [11]. Finally, the proposed fluorine-based chemical method converts Al, Li and K into sulfates as reaction 3 to maximize the leaching efficiency of Li and efficient consumption of HF.

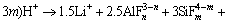
 (n: 0-6; m: 1-6) (1)
(n: 0-6; m: 1-6) (1)
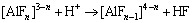 (2)
(2)
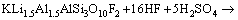

 (3)
(3)
Figure 1 shows the schematic process to extract Li from lepidolite using HF/H2SO4. 98% of Li was efficiently leached under investigated optimal conditions: ore/HF/H2SO4ratio of 1: 2: 3.5 (g/mL/mL) at 85°C for 3h [8]. The relatively selective leaching of Li over Si was received due to the lepidolite preferentially dissolved over quartz, which is important for the downstream purification and separation.
As for acidizing in the petrochemical industry, the dissolving of sandstones in HF/HCl system (called mud acid) was considered that the surficial adsorption of HF molecules was the rate-limiting step [22-24]. In this work, the leaching kinetics of Li from lepidolite has been discussed in the mixture of sulfuric acid and hydrofluoric acid (H2SO4/HF). The compositions of lepidolite are significantly different from sandstones (which are mainly carbonate minerals), and the H2SO4 is less volatile than HCl, resulting in a different leaching behavior. Our previous work indicated that the HF/ore ratio showed significant effects on the leaching efficiency and the compositions of generated insoluble residues. Here, effects of different HF/ore ratio and leaching temperature on leaching kinetics of Li were carried out to further understand selective leaching of Li.

Figure 1 Schematic diagram of extract Li from lepidolite using H2SO4/HF as lixiviant
2 Experimental
2.1 Materials and reagents
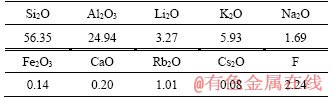
The lepidolite sampled from Yichun was accompanied with albite (NaAlSi3O8) and some quartz (SiO2). About 3.2% Li2O was contained in the concentrate. Other major elements analysis was determined and listed in Table 2. The ore sample was ground by planetary ball mill for 30 min for leaching experiments.
Table 2 Chemical analysis of lepidolite ore sample (mass fraction, %)
Except for the analytical grade hydrofluoric acid (40 wt%), 1:1 (V/V) diluted sulfuric acid was added to convert Li, Al and K into sulfates because the concentrated H2SO4 (98 wt%) presents more oxidative and too viscous for H+ transportation.
2.2 Procedures and methods
The leaching system was typically set up in a 100 mL Teflon crucible reactor with an oil bath as heating system. The ore sample, pre-wetted with certain amount of deionized water, was then mixed with 1:1 H2SO4 under the optimal conditions. The hydrofluoric acid was immediately added once the desired temperature was reached (50-85°C). Then the slurry kept stirred continuously at 150 r/min for different leaching time. The resulted slurry was separated and analyzed to obtain series of kinetics data. The kinetics data were valued as averaged by repeating three times. The leaching efficiency of Li (L) and the insoluble residues ratio (R) were introduced to evaluate the efficiency of leaching process. The retention percentage of F (Frent,l) in the leaching solution was also introduced.
Elemental analysis along with some characterization such as XRD (X-ray diffraction), FTIR (Fourier-transform infrared spectroscopy) and NMR (Nuclear magnetic resonance) were employed to determine the compositions of leaching solution and insoluble residues. Detailed instrument information has presented in our previous work [8].
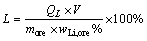 (4)
(4)
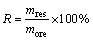 (5)
(5)
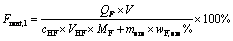 (6)
(6)
where QL and QF are mass concentrations of Li and F in leaching solution, respectively, g/L; V and VHF are volumes of leaching solution and the added HF, respectively, L; more is mass of lepidolite ore sample, g; cHF is concentration of the HF added, mol/L; wLi,ore and wF,ore are mass fractions of Li and F in the lepidolite, respectively, %.
3 Results and discussion
3.1 Investigation of leaching kinetics
Effects of different leaching conditions have been discussed in our previous work [8], such as HF/ore ratio (1:1-3.5:1 mL/g), H2SO4/ore ratio (2:1-4:1 mL/g), leaching temperature (50-100°C) and leaching time (2-4 h). The results showed that the added amount of HF (HF/ore) and leaching temperature are the crucial factor that significantly influenced on the leaching process. The introduced H+ was employed to convert insoluble residues into soluble sulfates. However, the added H2SO4 shows slight effects on leaching efficiency of Li under the investigated range, which can be verified by XRD analyses of insoluble residues in our previous work [8]. The leaching solution is strongly acidic under the investigated conditions with a negative pH value without specific meaning (pH -0.5). However, the pH is very important for Al removal during separation and purification, which will be discussed in detailed in our future works.
The leaching kinetics of Li in Figure 2 was investigated under different leaching temperatures (50-85°C) with an optimal ore/HF/H2SO4 ratio of 1: 2: 3.5 (g/mL/mL) and stirred at 150 r/min. The distribution curve of particle size was showed in Figure 3(a). Moreover, effect of HF/ore ratio and leaching temperature on leaching of Li, Al and Si were also investigated, aiming to further understand the selective leaching behavior of lepidolite.
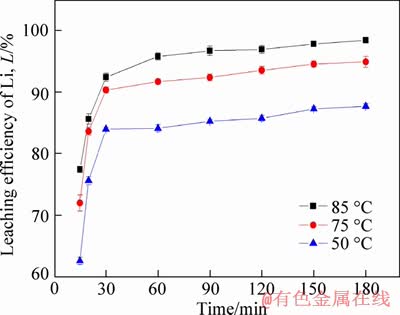
Figure 2 Leaching kinetics of lithium from lepidolite in HF/H2SO4 under different leaching temperatures
The shrinking-core model, which is commonly applied in leaching system or hydrometallurgy fields, is employed to analyze the kinetics data with the ore particles treated as spherical particles. Our previous work indicated that insoluble products like amorphous SiO2 and AlF3 were generated on the surface of minerals [8]. The insoluble fluorides can be further dissolved by the introduced H+. Different equations were employed to analyze the kinetics data according to different rate-limiting steps theories [25-31].
Chemical reaction (Eq. (7)):
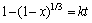 (7)
(7)
Inner diffusion (Eq. (8)):
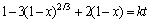 (8)
(8)
Mixed controlling process (Eq. (9)):
 (9)
(9)
However, the fitting results showed that none of these three equations could fit well with the whole leaching process. The leaching process was then separated into two periods due to that the L reached 85% for 30 min. The results in Figure 3(b) showed that the chemical reaction and inner diffusion were the limiting step of leaching Li at the beginning of leaching (0-30 min) with apparent energy (Ea) calculated as 20.62 kJ/mol (Figure 3(c)). While as for the leaching (60-180 min) continues, the inner diffusion became as the rate-limiting step that the Li+ diffused through solid product layers with Ea calculated as 19.37 kJ/mol (Figure 3(d)).
The leaching rate of Li during 0-30 min, which was controlled by the surface chemical reaction, could be attributed to the protonation attack of HF and H+. With the reaction proceeding, the insoluble products resulted in the inner diffusion leaching as rate-limiting step. This kinetics of leaching Li from lepidolite in HF/H2SO4 was different from that of α-spodumene [30], which has a higher Ea due to the lower chemical reactivity of α-spodumene.
3.2 Leaching of Li, Al and Si
The HF/ore and leaching temperature show obvious effect on leaching efficiency. Therefore, their effects on the leaching of Li, Al and Si were carried out to further understand the selective leaching behavior of lepidolite.
3.2.1 Effect of HF/ore
Figure 4 indicates that the HF/ore obviously influenced the leaching process that the leaching efficiency of Li, Al and Si increased dramatically with the increasing of HF/ore ratio, especially for the L.
3.2.2 Effect of leaching temperature
Figure 5 indicates that the leaching temperature affects the leaching of Li more obviously than that of Al and Si, due to that Al and Si formed insoluble products like AlF3 and SiO2 while Li mainly existed as soluble Li2SO4.
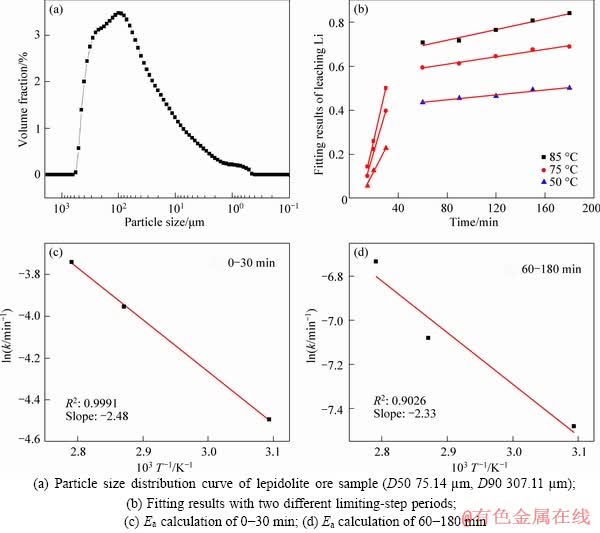
Figure 3 Kinetics investigation of leaching Li from lepidolite:
3.3 F retention in leaching system
3.3.1 Elemental analysis of leaching solution
The elemental analysis of leaching solution in Table 3 shows that 98% of Li can be leached along with more than 90% of Al and other metal elements (except K, which remained as insoluble K2SiF6) under the optimal leaching conditions (ore/HF/ H2SO4 ratio of 1: 2: 3.5 g/mL/mL at 85 °C for 3 h). However, considerable F was contained in leaching solution, which should be removed or recovered with subsequent process. Here, effects of different HF/ore ratio and leaching temperature on F retention were discussed. XRD and FTIR were employed to further determine the existing forms of fluorine in insoluble residues. As for leaching solution, chemical environment of typical element 27Al and 19F was analyzed by NMR to reveal the existing form of F, which is important for further removal or recovery of F.
3.3.2 Effect on F retention in leaching solution
The results in Figure 6 showed that the F retention reached above 90% under the investigated
conditions, which is important for efficient using of F. The investigation also showed that the higher F retention was achieved at lower HF/ore due to the efficient conversion from fluorides into sulfates by H+. The higher leaching temperature is benefit for converting fluorides into sulfates. Considering that the crucial effect of HF/ore on leaching, the leaching is conducted with the HF/ore ratio of 2:1 (mL/g) at 85 °C.
3.3.3 27Al and 19F NMR analysis of leaching solution
Considering that the Al3+ can easily form coordination with F-, the existing form of F in the liquid phase is important for subsequent separation and purification. Therefore, typical elements of 27Al and 19F were investigated using NMR to determine the existing form of F.
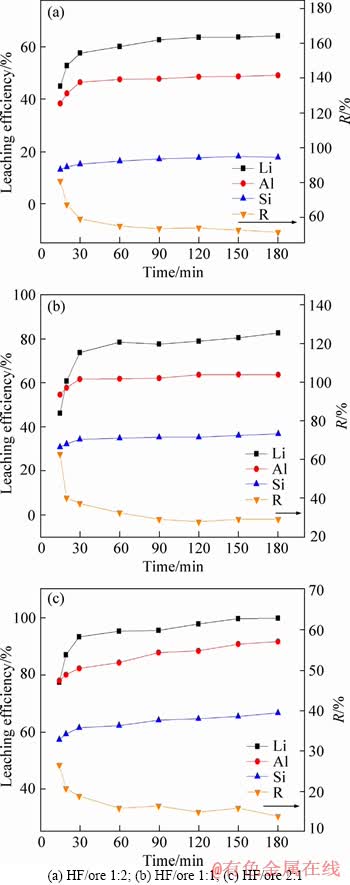
Figure 4 Effect of HF/ore on leaching of Li, Al and Si:
Based on our previous NMR analysis of liquid phase compositions of spodumene dissolved in HF/H2SO4 [30], it can be concluded that the existing forms of Al and F in the liquid phase were determined by the complexation equilibrium between Al and F. The Al and F can exist as M3[AlF6] or [AlFn]3-n, n=1, 2, 3, …, 6 [32-34] or even complexing with other metal elements. Thus,the NMR analysis of existing form of F is important for understand the dissolution behavior.
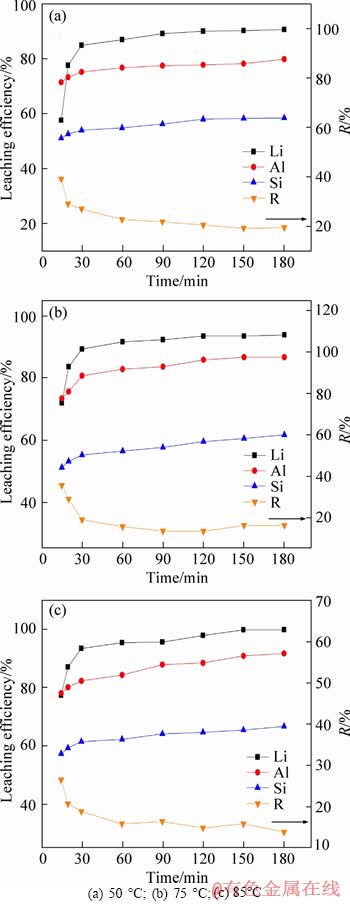
Figure 5 Effect of leaching temperature on difference among leaching of Li, Al and Si:
More importantly, the introduced H+ affects the complexing equilibrium between Al and F. 27Al NMR analysis in Figure 7 of the leaching solution of lepidolite shows that Al mainly exists in two forms: four-coordinated aluminium (AlIV, ~80×10-6) and six-coordinated aluminium (AlVI, ~0). The slight shifting is mainly due to the exchange among different ligands. Because F- as ligand can exchange rapidly with different ligands such as H3O+ since F as the most negative non-metallic element. Combined with the analysis of 19F NMR signal in Figure 8, the signal near -129.00×10-6 may belong to F-/HF and SiF62- chemical shift, and the chemical shift around -150.41×10-6 belongs to the resonance signal formed by four-coordinate complexation between AlIV and F, while the -156×10-6 can be attributed to the six-coordination complexation between AlVI and F [32-34]. The NMR analysis results showed that the AlIV-F in 15 min was decreased compared with 180 min, indicating insoluble Al-F fluoride was gradually dissolved with leaching proceeding.
Table 3 Elemental analysis of leaching solution and insoluble residues under optimal conditions
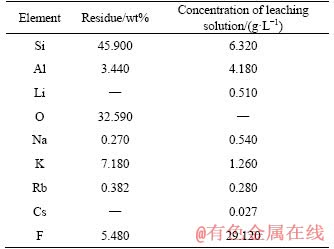
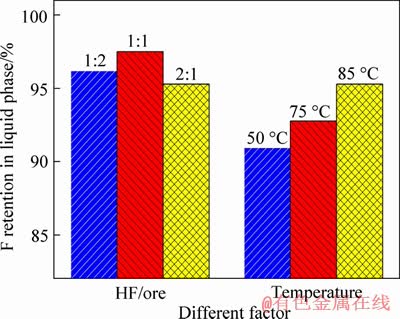
Figure 6 Effect of HF/ore and leaching temperature on F retention in the liquid phase
The 27Al and 19F NMR analysis of the leaching solution showed that AlVI and F respectively existed as Al3+ and F-/HF, which can be confirmed with the subsequent analysis of corresponding residues.
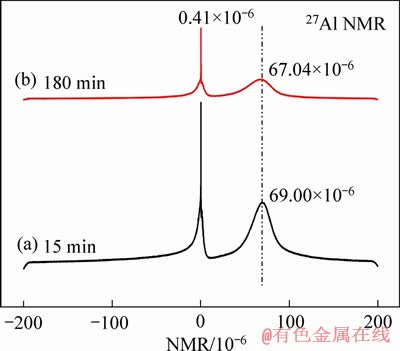
Figure 7 27Al NMR spectra of leaching solution of kinetics at 85 °C
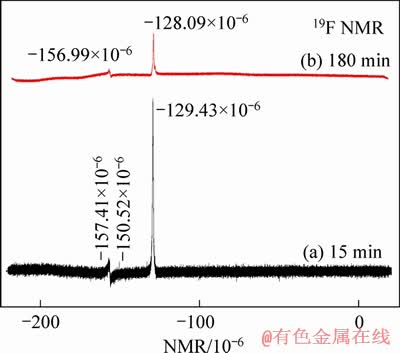
Figure 8 19F NMR spectra of leaching solution of kinetics at 85 °C
3.3.4 XRD analysis of insoluble residues
The XRD analysis in Figure 9 indicated that the characteristic peaks of albite and lepidolite were gradually decreased. Characteristic peaks of generated K2SiF6 (PDF#07-0217) were obviously detected. However, the quartz remained during the leaching process, resulting in an incongruent leaching of Li over Si, which is important for selective leaching of Li from lepidolite. However, no coordinated products between Al and F or even with Li were detected by XRD. Then, FTIR was carried out to further determine the composition of insoluble residues.
3.3.5 FTIR analysis of insoluble residues
The FTIR analysis in Figure 10 of lepidolite ore sample and insoluble residues showed that the M-O (M: Li, Al, K … metal elements) stretching vibration absorption peaks at 482.42 cm-1 in the near infrared region <500 cm-1 was obviously decreased during the leaching, indicating that the metal elements Li, K, Na and Al were efficiently leached. The absorption peaks of 694.81 cm-1 occurred due to the stretching vibration of Al-F and the stretching vibration absorption peaks of Si—F—Si at 771.03 cm-1 were detected [35]. The peaks at 1071.81 cm-1 belonging to the stretching vibration peak of Si—O always remained during leaching process, indicating that a relatively selectively leaching of Li, Al over Si resulted in this fluorine-based acid treatment using HF/H2SO4.
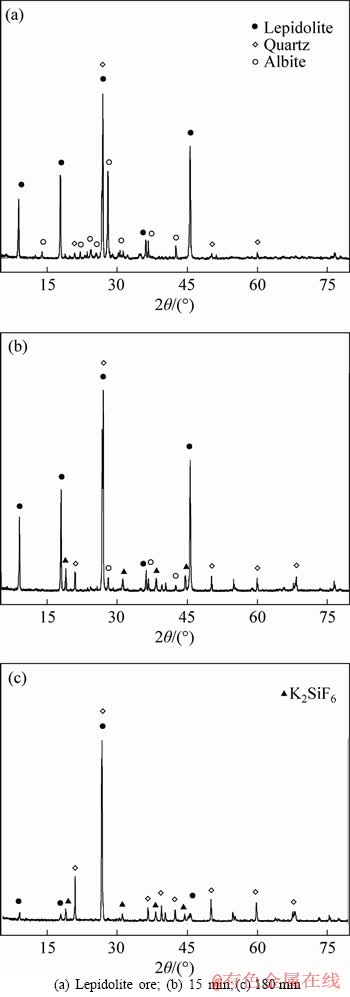
Figure 9 XRD analyses of insoluble residues obtained from kinetics at 85°C:
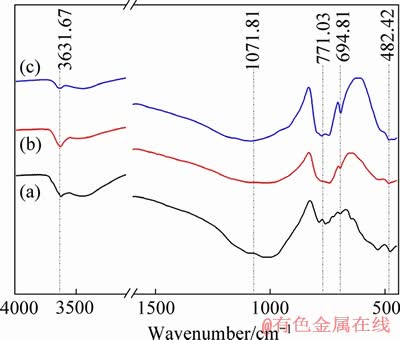
Figure 10 FTIR analyses of insoluble residues obtained from kinetics at 85°C
Combined with the analysis of the corresponding leaching solution, the F in solid phase was insoluble fluorides Al-F and K2SiF6. These insoluble residues formed solid layers which affected the leaching rate of Li, which somehow resulted in this selective and incongruent leaching behavior of lepidolite.
4 Conclusions
1) The leaching kinetics of Li indicated that the chemical reaction and inner diffusion were rate-limiting step at the early stage of the leaching (0-30 min) at 50-85 °C, with an apparent energy Ea of 20.62 kJ/mol. The inner diffusion through solid layers became the rate-limiting step with leaching continues.
2) The HF/ore ratio and leaching temperature had an obvious effect on selective leaching Li over Al and Si.
3) More than 90% of fluorine was remained in leaching solution as HF/F-, which is important for further utilization of F. The F in the solid phase mainly existed as insoluble AlF3·3H2O and K2SiF6.
Acknowledgments
The authors also thank for the Changsha Research Institute of Mining and Metallurgy for elemental analyses.
References
[1] CHOUBEY P K, KIM M S, SRIVASTAVA R R, LEE J C, LEE J Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources [J]. Minerals Engineering, 2016, 89: 119-137. DOI: 10.1016/j.mineng.2016.01.010.
[2] MESHRAM P, PANDEY B D, MANKHAND T R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review [J]. Hydrometallurgy, 2014, 150: 192-208. DOI: 10.1016/j.hydromet.2014.10.012.
[3] KESLER S E, GRUBER P W, MEDINA P A, KEOLEIAN G A, EVERSON M P, WALLINGTON T J. Global lithium resources: Relative importance of pegmatite, brine and other deposits [J]. Ore Geology Reviews, 2012, 48(5): 55-69. DOI: 10.1016/j.oregeorev.2012.05.006.
[4] LI Huan, EKSTEEN J, KUANG Ge. Recovery of lithium from mineral resources: State-of-the-art and perspectives–A review [J]. Hydrometallurgy,2019, 189: 105129. DOI: 10.1016/j.hydromet.2019.105129.
[5] KUANG Ge, LIU Yu, LI Huan, XING Sheng-zhou, LI Fu-jie, GUO Hui. Extraction of lithium from β-spodumene using sodium sulfate solution [J]. Hydrometallurgy, 2018, 177: 49-56. DOI: 10.1016/j.hydromet.2018.02.015.
[6] GUO Hui, KUANG Ge, WANG Hai-dong, YU Hai-zhao, ZHAO Xiao-kang. Investigation of enhanced leaching of lithium from α-spodumene using hydrofluoric and sulfuric acid [J]. Minerals, 2017, 7(11): 205. DOI: 10.3390/ min7110205.
[7] MARTIN G, SCHNEIDER A, VOIGT W, BERTAU M. Lithium extraction from the mineral zinnwaldite: Part II: Lithium carbonate recovery by direct carbonation of sintered zinnwaldite concentrate [J]. Minerals Engineering, 2017, 110: 75-81. DOI: 10.1016/j.mineng.2017.04.009.
[8] GUO Hui, KUANG Ge, WAN Hao, YANG Yi, YU Hai-zhao, WANG Hai-dong. Enhanced acid treatment to extract lithium from lepidolite with a fluorine-based chemical method [J]. Hydrometallurgy, 2019, 183: 9-19 DOI: 10.1016/j.hydromet. 2018.10.020.
[9] KUANG Ge, LI Huan, HU Song, JIN Ran, LIU Shan-jun, GUO Hui. Recovery of aluminium and lithium from gypsum residue obtained in the process of lithium extraction from lepidolite [J]. Hydrometallurgy, 2015, 157: 214-218. DOI: 10.1016/j.hydromet.2015.08.020.
[10] GUO Hui, KUANG Ge, YANG Jing-xi, SONG Hu. Fundamental research on a new process to remove Al3+ as potassium alum during lithium extraction from lepidolite [J]. Metallurgical and Materials Transactions B, 2016, 47: 3557–3564. DOI: 10.1007/s11663-016-0774-y.
[11] HEKIM Y, FOGLER H S. Acidization-VI on the equilibrium relationships and stoichiometry of reactions in mud acid [J]. Chemical Engineering Science, 1977, 32: 1-9. DOI: 10.1016/0009-2509(77)80188-7.
[12] OGORODOVA L P, KISELEVA I A, MELCHAKOVA L V, SCHURIGA T N. Thermodynamic properties of lithium mica: Lepidolite [J]. Geochemistry International, 2010, 435(1): 68-70. DOI: 10.1016/j.tca.2005.04.026.
[13] VIECELI N, NOGUEIRA C A, PEREIRA M F C, DIAS A P S, DURAO F O, GUIMARAES C, MARGARIDO F. Effects of mechanical activation on lithium extraction from a lepidolite ore concentrate [J]. Minerals Engineering, 2017, 102: 1-14. DOI: 10.1016/j.mineng.2016.12.001.
[14] LUONG V T, KANG Dong-jun, AN J W, DAO D A , KIM M J, TRAN T. Iron sulphate roasting for extraction of lithium from lepidolite [J]. Hydrometallurgy, 2014, 141: 8-16. DOI: 10.1016/j.hydromet.2013.09.016.
[15] YAN Qun-xuan, LI Xin-hai, YIN Zhou-lan, WANG Zhi-xing, GUO Hua-jun, PENG Wen-jie, HU Qi-yang. A novel process for extracting lithium from lepidolite [J]. Hydrometallurgy, 2012, 121-124: 54-59. DOI: 10.1016/j.hydromet.2012.04. 006.
[16] YAN Qun-xuan, LI Xin-hai, WANG Zhi-xing, WANG Jie-xi, GUO Hua-jun, HU Qi-yang, PENG Wen-jie, WU Xi-fei. Extraction of lithium from lepidolite using chlorination roasting–water leaching process [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 1753-1759. DOI: 10.1016/S1003-6326(11)61383-6.
[17] HIEN-DINH T T, LUONG V T, GIERE R, TRAN T. Extraction of lithium from lepidolite via iron sulphide roasting and water leaching [J]. Hydrometallurgy, 2015, 153: 154-159. DOI: 10.1016/j.hydromet.2015.03.002.
[18] LEE J. Extraction of lithium from lepidolite using mixed grinding with sodium sulfide followed by water leaching [J]. Minerals, 2015, 5: 737-743. DOI: 10.3390/min5040521.
[19] YAN Qun-xuan, LI Xin-hai, WANG Zhi-xing, WU Xi-fei, GUO Hua-jun, HU Qi-yang, PENG Wen-jie, WANG Jie-xi. Extraction of valuable metals from lepidolite [J]. Hydrometallurgy, 2012, s117-118: 116-118. DOI: 10.1016/ j.hydromet.2012.02.004.
[20] ROSALES G D, DEL CARMEN RUIZ M, RODRIGUEZ M H. Novel process for the extraction of lithium from β-spodumene by leaching with HF[J]. Hydrometallurgy, 2014, 147-148: 1-6. DOI: 10.1016/j.hydromet.2014.04.009.
[21] REICHEL S, AUBEL T, PATZIG A, JANNECK E, MARTIN M. Lithium recovery from lithium-containing micas using sulfur oxidizing microorganisms [J]. Minerals Engineering, 2017, 106: 18-21. DOI: 10.1016/j.mineng.2017.02.012.
[22] FOGLER H S, LUND K, MCCUNE C C. Acidization III—The kinetics of the dissolution of sodium and potassium feldspar in HF/HCl acid mixtures [J]. Chemical Engineering Science, 1975, 30(11): 1325-1332. DOI: 10.1016/0009- 2509(75)85061-5.
[23] LI Nian-yin, ZENG Fan-hua, LI Jun, ZHANG Qian, FENG Yan-lin, LIU Ping-li. Kinetic mechanics of the reactions between HCl/HF acid mixtures and sandstone minerals [J]. Journal of Natural Gas Science and Engineering, 2016, 34: 792-802. DOI: 10.1016/j.jngse.2016.07.044.
[24] KLINE W E, FOGLER H S. Dissolution kinetics: Catalysis by strong acids [J]. Journal of Colloid and Interface Science, 1981, 82(1): 93-102. DOI: 10.1016/0021-9797(81)90127-2.
[25] TIAN Jun, YIN Jing-qun, CHI Ruan, RAO Guo-hua, JIANG Min-tao, OUYANG Ke-xian. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution [J]. Hydrometallurgy, 2010, 101(3, 4): 166-170. DOI: 10.1016/j.hydromet.2010.01.001.
[26] DICKINSON C F, HEAL G R. Solid–liquid diffusion controlled rate equations [J]. Thermochimica Acta, 1999, 340-341: 89-103. DOI: 10.1016/s0040-6031(99)00256-7.
[27] ZHAO Xun, YANG Jing, MA Hong-wen, LIU Mei-tang, LIN Fei. Kinetics of lepidolite decomposition reaction in sulfuric acid solution [J]. Chinese Journal of Nonferrous Metals, 2015, 25(9): 2588-2595. DOI: 10.19476/j.ysxb.1004. 0609.2015.09.035. (in Chinese)
[28] HUANG Yu-kun, DOU Zhi-he, ZHANG Ting-an, LIU Jiang. Leaching kinetics of rare earth elements and fluoride from mixed rare earth concentrate after roasting with calcium hydroxide and sodium hydroxide [J]. Hydrometallurgy, 2017, 173: 15-21. DOI: 10.1016/j.hydromet.2017.07.004.
[29] LIU Jia-nan, ZHAI Yu-chun, WU Yan, ZHANG Jun, SHEN Xiao-yi. Kinetics of roasting potash feldspar in presence of sodium carbonate [J]. Journal of Central South University, 2017, 24(7): 1544-1550. DOI: 10.1007/s11771-017-3559-9.
[30] GUO Hui, YU Hai-zhao, ZHOU An-an, LU Meng-hua, WANG Qiao, KUANG Ge, WANG Hai-dong. Kinetics of leaching lithium from α-spodumene in enhanced acid treatment using HF/H2SO4 as medium [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(2): 407-415. DOI: 10.1016/S1003-6326(19)64950-2.
[31] CHEN Bing, SHEN Xiao-yi, GU Hui-min, SHAO Hong-mei, ZHAI Yu-chun, MA Pei-hua. Extracting reaction mechanism analysis of Zn and Si from zinc oxide ore by NaOH roasting method [J]. Journal of Central South University, 2017, 24: 2266-2274(2017). DOI:10.1007/s11771-017-3637-z.
[32] MARTINEZ E J, GIRARDET J L, MORAT C. Multinuclear NMR study of fluoroaluminate complexes in aqueous solution [J]. Inorganic Chemistry, 1996, 35(3): 706-710. DOI: 10.1021/ic9507575.
[33] DUKE C V A, MILLER J M, CLARK J H, KYBETT A P. 19F mas NMR and FTIR analysis of the adsorption of alkali metal fluorides onto alumina [J]. Journal of Molecular Catalysis, 1990, 62(2): 233-242. DOI: 10.1016/0304- 5102(90)85216-5.
[34] FINNEY W F, WILSON E, CALLENDER A, MORRIS M D, BECK L W. Reexamination of Hexafluorosilicate Hydrolysis by 19F NMR and pH measurement [J]. Environmental Science & Technology, 2006, 40(8): 2572-2577. DOI: 10.1021/es052295s.
[35] HU Pei-wei, YANG Hua-ming. Insight into the physicochemical aspects of kaolins with different morphologies [J]. Applied Clay Science, 2013, 74: 58-65. DOI: 10.1016/j.clay.2012.10.003.
(Edited by HE Yun-bin)
中文导读
锂云母混酸HF/H2SO4浸出锂的动力学研究
摘要:锂云母混酸HF/H2SO4浸出动力学研究表明:50~85 °C下锂浸出速率在浸出前期(0~30 min)由表面化学反应以及内扩散共同控制,表观活化能Ea为20.62 kJ/mol;浸出后期(60~180 min)则主要由产物内扩散控制。氢氟酸添加量相比浸出温度对Li、Al和Si的浸出速率影响更显著。浸出温度对Li浸出效率影响比对Al和Si的影响更明显。F元素在固相不溶渣中的存在形式则主要为Al-F不溶氟化物和K2SiF6。此外,液相中F元素在所研究的氢氟酸添加量及浸出温度下均可保持较高保留率(>90%),为F元素的高效利用及后续回收利用提供保障。
关键词:锂云母;矿物提锂;氟化学法;氢氟酸;选择性浸出;动力学
Foundation item: Project(51474237) supported by the National Natural Science Foundation of China
Received date: 2019-04-08; Accepted date: 2019-12-20
Corresponding author: GUO Hui, PhD; Tel: +86-731-88879622; E-mail: hnhguo89@126.com; ORCID: 0000-0002-3554-7212