DOI: 10.11817/j.ysxb.1004.0609.2020-36467
废旧三元电池正极活性材料酸性浸出液中钴镍锰锂的分离与回收
蒋 玲1,詹 路1, 2,张秋卓1, 3
(1. 华东师范大学生 态与环境科学学院,上海 200241;
2. 上海交通大学 环境科学与工程学院,上海 200240;
3. 崇明生态研究院,上海 200062)
摘 要:废旧三元电池正极活性材料中大部分固态粉末的金属离子可以通过硫酸浸取剂转移至酸溶液中。将富集在溶液中的金属离子分离对废旧三元电池中有价金属的回收具有重大的意义。本文研究硫酸浸出液中钴、镍、锰、锂的分离与回收。提出的沉淀工艺流程为草酸铵沉淀法沉淀钴-碳酸氢铵沉淀法沉淀锰-碳酸钠氢氧化钠共沉淀法沉淀镍-碳酸钠沉淀法沉淀锂。通过改变溶液pH值、温度、搅拌时间、沉淀剂用量等参数,优化钴镍锰锂的分离条件。结果表明:在优化工艺条件下,溶液中的金属离子分别以草酸钴、碳酸锰、碱式碳酸镍、碳酸锂的形式沉淀,钴锰镍锂回收率分别达到99.17%、97.88%、93.47%、85.21%,产物草酸钴、碳酸锰、碱式碳酸镍、碳酸锂的质量分数分别为99.87%、98.89%、98.46%、96.52%。
关键词:三元锂电池;硫酸浸出液;钴镍锰锂;沉淀分离
文章编号:1004-0609(2020)-11-2684-11 中图分类号:X705 文献标志码:A
随着科学技术的发展,锂离子电池作为一种集高能量密度和高电压为一体的储能装置,广泛应用于移动和无线电子设备、电动工具、混合动力和电动交通工具等领域。当前车用动力电池基本以锂离子电池为主,据统计,全球锂离子电池消费量从2000年的5亿只迅速上升到2015年的70亿只[1-2]。锂离子电池的使用寿命一般3~5年,所以废旧锂离子电池数量也在逐年急剧增加[3]。预计2020年后,大量的动力电池将被淘汰,仅我国废旧锂离子电池数量将会达到250亿只,总质量50万t[4]。废旧电池中的有毒有害物质一旦泄漏,进入土壤、水体和大气,就会造成严重污染,钴、镍、铜、铝、锰等金属还具有累积效应,通过食物链富集在人体,具有极大的危害性。因此,需对废旧锂离子电池进行集中无害化处理,回收其中的金属材料,确保人类的健康和环境的可持续发展。
根据中国有色金属工业协会锂业分会统计,2017年我国锂离子电池正极材料产量约32.3万t,同比增长49.54%。其中三元材料(LiNixCo1-x-yMnyO2,简称NMC)、磷酸铁锂(LiFePO4)和钴酸锂(LiCoO2)2017年的产量分别为12.6万t、10.1万t和6.0万t[5]。锂离子电池三元正极活性材料,含有大量的有价金属钴、镍、锰、锂。从废旧电池材料中回收这些有价金属,实现资源化,将产生显著的环境效益和经济效益,意义深远。废旧三元锂电池的正极活性材料中的金属回收方法主要有火法冶金和湿法冶金[6-8]。火法冶金通过高温处理直接提取电极中的金属或金属氧化物,工艺简单,但回收材料纯度低,反应过程中容易产生有害气体[9]。湿法冶金经过预处理、浸出、浸出液净化和金属提取等流程将有价金属富集在溶液中再进行分离,获得各金属相应的盐或氧化物[4]。湿法回收操作条件较火法冶金温和,并且金属回收率高,产物杂质少,成为目前国内外的研究热点[10]。针对三元材料有价金属浸取主要方法是酸浸法。无机酸能解离出氢离子,表现出较强的酸性,对Li、Co、Mn、Ni具有较强的浸取效果。常用的无机酸浸取剂主要是盐酸(HCl)[11]、硫酸(H2SO4)[12-14]、硝酸(HNO3)[15-17]等。钴、锂、镍、锰等金属均以离子形式存在于浸出液中,需通过进一步的深度处理,因此,对于浸出液中的各类金属分离回收的研究也是十分必要的。一般采取溶剂萃取法、化学沉淀法、电化学沉积法等方法实现有价金属的分离提取[18-20]。
萃取法是指选择一种特定的萃取剂或几种萃取剂的混合物,与目标金属离子形成稳定的配合物,配合物在有机萃取剂中与浸出液分开,再利用相应的溶剂将配合物中的金属离子反萃取出来,实现金属离子的分离提纯[21]。HONG等[22]选用PC-88A、cyanex272和D2EHPA作为萃取剂,分别对Co、Ni和Mn从浸取液中分离。萃取法的优点是选择性好,利用不同的萃取剂,可得到高浓度的目标金属离子溶液。但是,溶剂在萃取过程中也会有一定的流失,而且萃取剂的价格较高,使得该方法在金属回收方面有一定的局限[23]。化学沉淀法是向金属浸出液中加入适当沉淀剂,使之发生反应并产生沉淀从而实现金属离子分离的一种方法[24]。常用的沉淀剂有氢氧化钠、草酸铵、草酸、碳酸钠等。CHEN等[25]先采用柠檬酸和葡萄糖将金属浸取,然后依次加入DMG(C4H8N2O2)、H2C2O4、H3PO4沉淀剂将Ni、Co、Li有价金属以Ni(C4H6N2O2)2、CoC2O4·2H2O、Li3PO4沉淀物形式分离,在最佳条件下,回收率分别达到98%、97%和89%。化学沉淀法的优势是设备要求低、成本低、操作简便等优点,将溶液中金属离子进行分步沉淀,得到各级分离的金属沉淀物,实现分离。但同样也存在缺点,由于浸出液中含有多种金属离子,易出现共沉淀的现象,难以分离。所以,简化操作程序、选择合适的沉淀剂、设定有效分离金属离子的工艺流程是很有必要的。
本文选取废旧三元锂电池的硫酸浸出液作为研究对象,选用不同的沉淀剂,对溶液中的钴、镍、锰、锂进行沉淀分离。研究了不同的沉淀条件下各金属的沉淀率,对每一工艺阶段获得的沉淀物进行提纯,希望获得纯净的金属化合物。探索了一条分离效率高、回收利用率大的废旧三元锂离子电池酸性浸出液中金属的回收工艺。
1 实验
1.1 实验原料
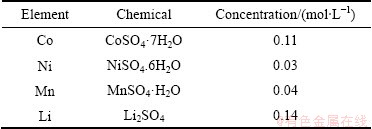
根据Meshram等在优化条件下得到的废旧三元锂电池酸性浸出料液的成分[26],配制了模拟料液。用CoSO4·7H2O、NiSO4.6H2O、MnSO4·H2O、Li2SO4配制硫酸浸出液模拟料液,料液中各金属含量见表1。
表1 废旧三元电池正极活性材料酸性浸出液模拟料液配制所用试剂及组成
Table 1 Reagents and composition of waste ternary battery positive active material acidic leaching solution
1.2 实验试剂
实验采用的沉淀剂有草酸铵((NH4)2C2O4)、氢氧化钠(NaOH)和碳酸钠(NaCO3),镍、锰检测用到的丁二酮肟(C4H8N2O2)、过硫酸铵((NH4)2S2O2),由国药集团化学试剂有限公司提供。钴、锂检测用到的化学试剂4-(5-氯-2-吡啶偶氮)-1,3-二氨基苯(C11H10ClN5)、钍试剂(C16H11AsN2Na2O10S2)由东京化成工业株式会社提供。
分别采用HCl-NaAc、NaAc-Hac、KH2PO4-NaOH、Na2CO3-NaHCO3、Na2HPO4-NaOH等缓冲溶液调节pH值(1.09~12.0)。用碳酸钠沉淀锂时,溶液用0.1 mol/L的氢氧化钠调节pH值。
1.3 实验方法
1) 检测方法
溶液中的钴采用4-(5-氯-2-吡啶偶氮)-1,3-二氨基苯分光光度法测定,标线为y=0.8750x-0.0070 (R2=1);溶液中的镍采用丁二酮肟分光光度法测定,标线为y=9.1936x-0.0044 (R2=1);溶液中的锰采用过硫酸铵分光光度法测定,标线为y=23.14x+0.0090 (R2=1);溶液中的锂采用钍试剂分光光度法测定,标线为y=5.0726x-0.0086 (R2=1)。沉淀物中的金属含量经过微波消解后采用等离子发射光谱仪测定。
2) 沉淀分离方法
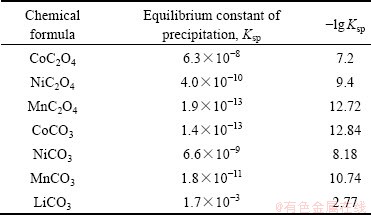
根据溶度积常数(Ksp)(见表2)以及金属化合物的性质确定各金属的沉淀顺序,建立工艺路线(见图1)。利用各种金属的溶解度的不同采用分步沉淀法将钴镍锰锂分开。沉淀反应在搭建的水浴锅与磁力搅拌器和全自动滴定管组合装置上进行,过滤用水系滤膜(0.22 μm)在砂芯过滤装置中抽滤。
3) 表征方法
回收产物用X射线荧光光谱仪确定其主要成分,采用冷场发射扫描电子显微镜观察其结构形态。
表2 常见钴镍锰锂化合物的溶度积常数表
Table 2 Table of solubility product constants of common cobalt nickel manganese lithium compounds
4) 实验参数
固定实验条件如下:Co2+、Ni2+、Mn2+、Li+的浓度为0.1 mol/L,草酸铵溶液为0.1 mol/L,碳酸氢铵为0.1 mol/L,碳酸钠溶液为0.1 mol/L,氢氧化钠为0.1 mol/L,改变pH值、反应温度、反应搅拌时间和沉淀剂加入量(De)与理论用量(Dt)之比等因素(见表3),研究硫酸体系中钴、镍、锰、锂的回收情况,并进行分析比较,优化条件寻求最佳分离条件。在最佳条件下分离废旧三元锂电池酸性浸出模拟料液,将每一沉淀过程中回收的沉淀物进行提纯,提纯方法见图2,回收各级分离的金属沉淀物,对回收产物进行表征。
表3 实验参数
Table 3 Experiment parameters


图1 钴镍锰锂沉淀分离工艺路线设计图
Fig. 1 Route design of cobalt-nickel-manganese-lithium precipitation separation process

图2 沉淀物提纯工艺图
Fig. 2 Diagram of precipitate purification process
2 结果与讨论
2.1 用草酸铵沉淀法从钴镍锰锂中分离钴
在含有钴镍锰锂的溶液中滴加3滴0.02 mol/L的氨水,采用反加法将溶液滴入草酸铵溶液中,磁力搅拌器转速设置为400 r/min,调控pH值、反应温度、反应搅拌时间、沉淀剂加入量4个条件,采用单因素实验法优化实验条件。少量的钴离子和镍离子与氨水反应生成络合物六氨合钴离子[Co(NH3)6]2+和六氨合镍离子[Ni(NH3)6]2+,有研究结果表明[Ni(NH3)6]2+比[Co(NH3)6]2+稳定性强[27],溶液中大量的镍与氨水络合反应,少量镍与离子与草酸根反应生成草酸镍沉淀,主要的沉淀反应如下:
Co2++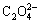 →CoC2O4↓ (1)
→CoC2O4↓ (1)
Ni2++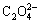 →NiC2O4↓ (2)
→NiC2O4↓ (2)
Mn2++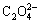 →MnC2O4↓ (3)
→MnC2O4↓ (3)
溶液静置0.5 h后用砂芯过滤装置对沉淀物与上清液进行分离,测定上清液中的钴镍锰锂离子含量,计算各金属的沉淀率。
在25 ℃、搅拌0.5 h、1倍的理论用量的草酸铵的条件下改变pH值,结果表明:随着pH值的升高,钴的沉淀率先升高后缓慢升高再快速降低;镍的沉淀率缓慢增加再缓慢降低;锰的沉淀率缓慢升高;锂的损失率较低,沉淀率在0.74%至1.03%之间(见图3(a))。pH值在2.0时,钴镍锰锂的沉淀率分别达到98.42%、11.78%、8.01%、0.98%,可以有效地沉淀分离钴。锂的沉淀率都较低,损失率可以忽略不计。
在pH=2.0、搅拌0.5 h、1倍的理论用量的草酸铵的条件下改变温度,结果表明:随着温度的升高,钴的沉淀率先升高后降低,镍和锰的沉淀率逐渐升高,锂的沉淀率变化不大,在0.99%至1.14%之间(见图3(b))。在55 ℃时,钴镍锰锂的沉淀率分别达到99.45%、19.18%、19.01%、1.11%,分离效果最好。
在pH=2.0、55 ℃、1倍的理论用量的草酸铵的条件下改变搅拌时间,结果表明:随着搅拌时间的增加,钴的沉淀率保持平缓;镍和锰的沉淀率持续增加;锂的沉淀率变化不大(见图3(c))。搅拌时间越长对钴的分在pH=2.0、55 ℃、搅拌1.0 h的条件下改变草酸铵的加入量,结果表明:随着沉淀剂实际用量的增加,钴的沉淀率缓慢降低;镍和锰的沉淀率持续增加;锂的沉淀率变化不大(见图3(d))。当草酸钴实际用量为理论用量的1.2倍时,分离效果最好。综合考虑,pH 2.0、温度 55℃、搅拌时间 1.0 h、沉淀剂用量为理论用量的1.2倍可作为最佳条件沉淀分离钴。
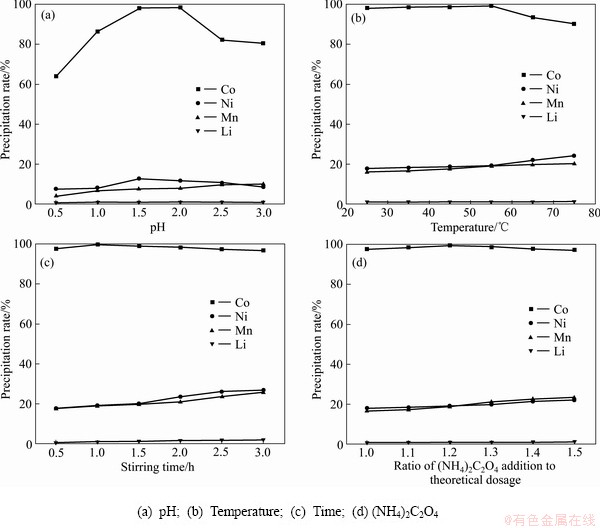
图3 不同条件下钴镍锰锂的沉淀率
Fig. 3 Precipitation rate of cobalt nickel manganese lithium under different conditions
2.2 用碳酸钠沉淀法从镍锰锂中分离锰
在含有镍锰锂的溶液中滴加3滴0.02 mol/L的氨水,采用反加法将溶液滴入碳酸氢铵溶液中,磁力搅拌器转速设置为400 r/min,调控pH值、反应温度、反应搅拌时间、沉淀剂加入量4个条件,采用单因素实验法优化实验条件。主要的沉淀反应如下:
Mn2++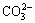 →MnCO3↓ (4)
→MnCO3↓ (4)
Li2++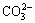 →Li2CO3↓ (5)
→Li2CO3↓ (5)
溶液静置0.5 h后用砂芯过滤装置对沉淀物与上清液进行分离,测定上清液中的镍锰锂离子含量,计算各金属的沉淀率。
离越不利,当搅拌时间达到1.0 h时,分离效果最好。
在25 ℃、搅拌0.5 h、1倍的理论用量的碳酸钠的条件下改变pH值,结果表明:随着pH值的升高,锰的沉淀率先升高后缓慢降低;镍的沉淀率先升高后降低;锂的沉淀率变化不大(见图4(a))。在pH值7.5时,锰镍锂的沉淀率分别达到92.66%、5.58%、0.07%,分离效果最好。
在pH=7.5、搅拌0.5 h、1倍的理论用量的碳酸钠的条件下改变温度,结果表明:随着温度的升高,锰的沉淀率持续增加;镍的不断增长;锂的沉淀率变化不大(见图4(b))。在45 ℃时,锰镍锂的沉淀率分别达到99.89%、8.77%、6.45%,分离效果最好。
在pH=7.5、45 ℃、1倍的理论用量的碳酸钠的条件下改变搅拌时间,结果表明:随着搅拌时间的增长,锰的沉淀率持续增长;镍的沉淀率下降;锂的沉淀率变化不大(见图4(c))。可见搅拌时间的增加可以减少镍的沉淀。搅拌时间达到2.5 h时分离效果最好。
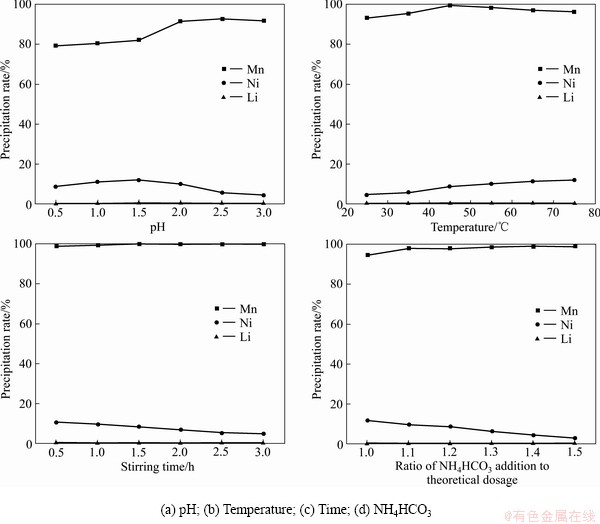
图4 不同条件下锰镍锂的沉淀率
Fig. 4 Precipitation rate of manganese nickel lithium under different conditions
在pH=7.5、45 ℃、搅拌2.5 h的条件下改变碳酸钠的加入量,结果表明:随着沉淀剂实际用量的增长,锰的沉淀率缓慢增长;镍的沉淀率逐渐降低,锂的沉淀率变化不大(见图4(d))。碳酸氢铵的实际用量是理论用量的1.3倍时沉淀分离锰效果最佳。综合考虑, pH 7.5、温度 45 ℃、搅拌时间 2.5 h、沉淀剂用量为理论用量的1.3倍可作为最佳条件沉淀分离锰。
2.3 用碳酸钠-氢氧化钠共沉淀法从镍锂中分离镍
将同等体积的0.02 mol/L的碳酸钠溶液和0.01 mol/L的氢氧化钠溶液混合,采用并流法将溶液滴入碳酸钠溶液中,磁力搅拌器转速设置为800 r/min,调控pH值、反应温度、反应搅拌时间、沉淀剂加入量4个条件,采用单因素实验法优化实验条件。主要的沉淀反应如下:
Ni2++2OH-+ →xNiCO3·yNi(OH)2↓ (6)
→xNiCO3·yNi(OH)2↓ (6)
溶液静置12 h后用砂芯过滤装置对沉淀物与上清液进行分离,测定上清液中的镍锂离子含量,计算镍和锂的沉淀率。
在25 ℃、搅拌0.5 h、1倍的理论用量的碳酸钠的条件下改变pH值,结果表明:随着pH值的升高,镍的沉淀率先升高后降低;锂的沉淀率在2%以下(见图5(a))。在pH值为10.0时,镍锂的沉淀率分别达到87.41%、1.65%。
在pH=9.5、搅拌0.5 h、1倍的理论用量的碳酸钠的条件下改变温度,结果表明:随着温度的升高,镍的沉淀率先升高后降低;锂的沉淀率在1%至2%之间 (见图5(b))。在55 ℃时,镍锂的沉淀率分别达到93.72%、1.72%。
在pH=9.5、65 ℃、1倍的理论用量的碳酸钠的条件下改变搅拌时间,结果表明:随着搅拌时间的升高,镍的沉淀率逐渐升高;锂的沉淀率在1%至2%之间(见图5(c))。在搅拌时间24 h时,镍锂的沉淀率分别达到93.74%、1.86%。
在pH=9.5、65 ℃、搅拌24 h的条件下改变碳酸钠的加入量,结果表明:随着碳酸钠实际用量的增加, 镍和锂的沉淀率都增加(见图5(d))。考虑到沉淀剂的消耗以及锂沉淀率的增高,选碳酸钠实际用量为理论用量的1.1倍作为最佳分离条件。综合考虑,pH 9.5、温度 65 ℃、搅拌时间 24 h、沉淀剂用量为理论用量的1.1倍可作为最佳条件沉淀分离镍。
2.4 用碳酸钠沉淀法沉淀锂
采用同加法将锂溶液与碳酸钠溶液滴入反应烧杯中,磁力搅拌器转速设置为400 r/min,调控pH值、温度、搅拌时间、沉淀剂加入量4个条件,采用单因素法优化条件。主要的沉淀反应如下:Li2++ → Li2CO3↓。溶液静置1.5 h后用砂芯过滤装置分离沉淀物与上清液,测定上清液中的锂离子含量,计算锂的沉淀率。
→ Li2CO3↓。溶液静置1.5 h后用砂芯过滤装置分离沉淀物与上清液,测定上清液中的锂离子含量,计算锂的沉淀率。
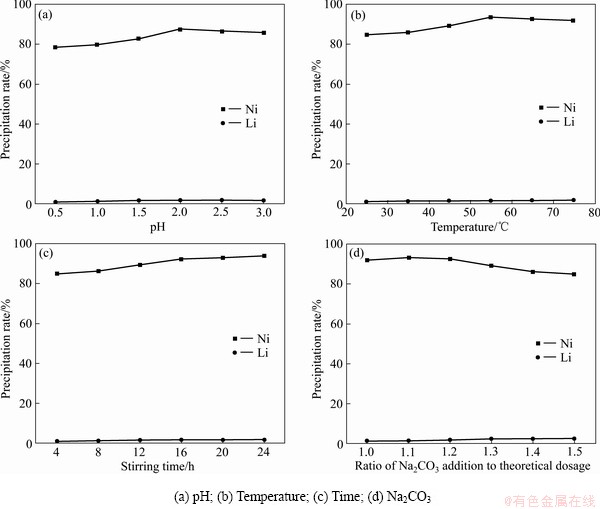
图5 不同条件下镍锂的沉淀率
Fig. 5 Precipitation rate of nickel-lithium under different conditions
在25 ℃、搅拌0.5 h、1倍的理论用量的碳酸钠的条件下改变pH值,结果表明:随着pH值的升高,锂的沉淀率升高。pH=14时,锂的沉淀率为72.89%(见图6(a))。在pH=14、搅拌0.5 h、1倍的理论用量的碳酸钠的条件下改变温度,结果表明:随着温度的升高,锂的沉淀率升高。65 ℃时,锂的沉淀率达到78.94%(见图6(b))。在pH=14、65 ℃、1倍的理论用量的碳酸钠的条件下改变搅拌时间,结果表明:锂的沉淀率随搅拌时间的增加而升高。搅拌4.0 h时,锂的沉淀率达到83.87%(见图6(c))。在pH=14、65 ℃、搅拌4 h的条件下改变碳酸钠的加入量,结果表明:随着碳酸钠实际加入量的增加,锂的沉淀率升高。当1.4倍理论用量的碳酸钠时,锂的沉淀率为85.18%(见图6(d))。因此,pH 14.0、65 ℃、搅拌时间4.0 h、1.4倍理论用量的碳酸钠可作为最佳条件沉淀锂。
2.5 优化条件下钴镍锰锂的沉淀分离与产物的表征
1) 优化条件下钴镍锰锂的沉淀分离
在优化条件下对料液中的金属进行分步回收:在pH 2.0、温度 55 ℃、搅拌时间 1.0 h、沉淀剂用量为理论用量的1.2倍条件下沉淀分离钴;过滤后,滤液中的剩余金属在pH 7.5、温度 45 ℃、搅拌时间 2.5 h、沉淀剂用量为理论用量的1.3倍的条件下沉淀分离锰;过滤后,滤液中的剩余金属在pH 9.5、温度 65 ℃、搅拌时间 24 h、沉淀剂用量为理论用量的1.1倍的条件下沉淀分离镍;过滤后,滤液中的剩余金属在pH 14.0、温度 65 ℃、搅拌时间 4.0 h、沉淀剂用量为理论用量的1.4倍的条件下沉淀锂。计算各个金属沉淀率可以得出,钴、锰、镍、锂的回收率分别达到98.99%、97.88%、93.47%、85.21%。与单因素实验结果对比,回收效果较为良好。在选择性沉淀分离过程中,依次获得产物草酸钴、碳酸锰、碱式碳酸镍、碳酸锂。在产物洗涤过程中,碱式碳酸镍用氨水洗涤,草酸钴、碳酸锰、碳酸锂采用超纯水洗涤。
2) 产物的表征
通过分析提纯产物的XRD谱确定其主要成分,通过电镜观察其主要形态。图7(a)和(a′)表明,第一步沉淀后的主要产物为粒径10 μm的二水合草酸钴,粉色固态沉淀。产物中的小块状团聚在大块状上呈现不规则块状。图7(b)和(b′)表明,第二步沉淀后的主要产物为粒径10 μm的球状碳酸锰,浅棕色固态沉淀。图7(c)和(c′)表明,第三步沉淀后的主要产物为粒径10 μm的松散的块状碱式碳酸镍,绿色固态沉淀。图7(d)和(d′)表明,第四步沉淀后的主要产物为粒径10 μm的块状碳酸锂,白色固态沉淀。
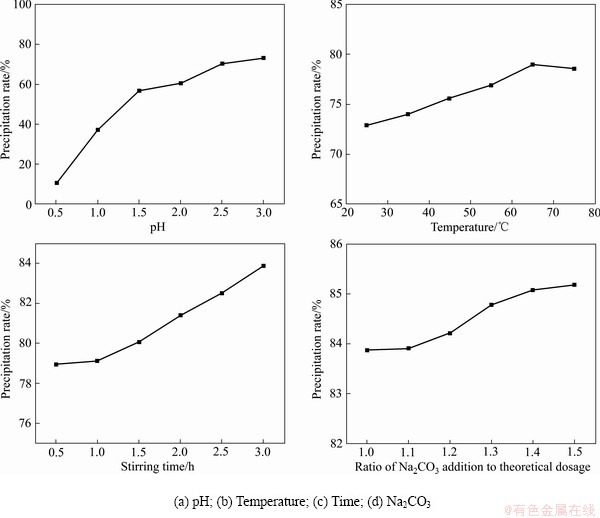
图6 不同条件下锂的沉淀率
Fig. 6 Precipitation rate of lithium under different conditions

图7 产物的XRD谱与SEM像
Fig. 7 XRD patterns and SEM images of products
3) 产物化学成分分析
量取0.05 g产物,经过微波消解后用ICP测定溶液中的金属离子含量,通过计算得出,产物草酸钴、碳酸锰、碱式碳酸镍、碳酸锂质量分数分别为99.87%、98.89%、98.46%、96.52%。产物中的金属离子的含量见表4。结果显示草酸钴沉淀物中镍锰锂含量较低;碳酸锰沉淀中钴镍锂含量较低;碱式碳酸镍沉淀物中钴锰锂含量较低;碳酸锂沉淀物中钴镍锰含量较低,杂质含量均在1%以下,可以忽略不计。因此该分离提纯工艺可以实现废旧锂电池正极材料中金属的资源化回收。
表4 回收产物的化学成分
Table 4 Chemical composition of product recovered

3 结论
1) 研究了废旧三元锂电池硫酸浸出液中的钴镍锰锂四种金属的分离回收,探索了一条操作简单回收效率高的工艺路线。
2) 草酸铵沉淀法沉淀钴-碳酸氢铵沉淀法沉淀 锰-碳酸钠氢氧化钠共沉淀法沉淀镍-碳酸钠沉淀法沉淀锂工艺可以有效分离酸性溶液中的钴镍锰锂,为溶液中的多金属分离提供了新方法。
3) 本工艺流程中所产生的废气无有毒成分,可直接外排,工艺废水中生成的NaSO4可回收利用。
REFERENCES
[1] XU J, THOMAS H R, FRANCIS R W, LUM K R, WANG J, LIANG B. A review of processes and technologies for the recycling of lithium-ion secondary batteries[J]. Journal of Power Sources, 2008, 177(2): 512-527.
[2] 李德耿, 吴 森, 夏 洋, 司士辉. 涂布法制备锌//聚苯胺二次电池[J]. 徐州工程学院学报(自然科学版), 2015, 30(1): 35-41.
LI De-geng, WU Sen, XIA Yang, SI Shi-hui. Preparation of zinc//polyaniline secondary battery by coating method[J]. Journal of Xuzhou Institute of Technology(Natural Science Edition), 2015, 30(1): 35-41.
[3] HE L P, SUN S Y, SONG X F, YU J G. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning[J]. Waste Management, 2015, 46: 523-528.
[4] 卫寿平, 孙 杰, 周 添, 李吉刚, 曹焕露. 废旧锂离子电池中金属材料回收技术研究进展[J]. 储能科学与技术, 2017, 6(6): 1196-1206.
WEI Shou-ping, SUN Jie, ZHOU Tian, LI Ji-gang, CAO Huan-lu. Research progress in metal materials recycling technology in waste lithium ion batteries[J]. Energy Storage Science and Technology, 2017, 6(6): 1196-1206.
[5] 黎华玲, 陈永珍, 宋文吉, 冯自平. 湿法回收退役三元锂离子电池有价金属的研究进展[J]. 化工进展, 2019, 38(2): 921-932.
LI Hua-ling, CHEN Yong-zhen, SONG Wen-ji, FENG Zi-ping. Research progress in the recovery of valuable metals from decommissioned ternary lithium ion batteries by wet process[J]. Chemical Progress, 2019, 38(2): 921-932.
[6] 张笑笑, 王鸯鸯, 刘 媛, 吴 锋, 李 丽. 废旧锂离子电池回收处理技术与资源化再生技术进展[J]. 化工进展, 2016, 35(12): 4026-4032.
ZHANG Xiao-xiao, WANG Yuan-yuan, LIU Yuan, WU Feng, LI Li. Progress in recycling technology and resource recycling technology for waste lithium ion batteries[J]. Chemical Industry and Engineering Progress, 2016, 35(12): 4026-4032.
[7] ZHANG T, HE Y, WANG F, GE L, ZHU X, LI H. Chemical and process mineralogical characterizations of spent lithium- ion batteries: An approach by multi analytical techniques[J]. Waste Management, 2014, 34(6): 1051-1058.
[8] NIE H, XU L, SONG D, SONG J, SHI X, WANG X, ZHANG L, YUAN Z. LiCoO2: recycling from spent batteries and regeneration with solid state synthesis[J]. Green Chemistry, 2015,17(2): 1276-1280.
[9] YANG Y, HUANG G Y, XU S M, HE Y H, LIU X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries[J]. Hydrometallurgy, 2016, 165: 390-396.
[10] SUN L, QIU K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management, 2012, 32: 1575-1582.
[11] MESHRAM P, ABHILASH, PANDEY B D, MANKHAND T R, DEVECI H. Acid baking of spent lithium ion batteries for selective recovery of major metals: A two-step process[J]. Journal of Industrial and Engineering Chemistry, 2016, 43: 117-126.
[12] 李 通. 锂、锰、稀土等有价金属分离、富集过程研究[D]. 兰州: 兰州大学, 2012.
LI Tong. Research on separation and enrichment process of valuable metals such as lithium, manganese and rare earth[D]. Lanzhou: Lanzhou University, 2012.
[13] 易爱飞, 朱兆武, 张 健, 苏 慧, 齐 涛. 废旧三元电池正极活性材料盐酸浸出液中钴锰共萃取分离镍锂[J]. 有色设备, 2018, 4: 4-9.
YI Ai-fei, ZHU Zhao-wu, ZHANG Jian, SU Hui, QI Tao. Separation of nickel and lithium by co-extraction of cobalt and manganese in hydrochloric acid leaching solution of waste ternary battery positive electrode[J]. Colored equipment, 2018, 4: 4-9.
[14] 陈 亮, 唐新村, 张 阳, 瞿 毅, 王志敏. 从废旧锂离子电池中分离回收钴镍锰[J]. 中国有色金属学报, 2011, 21(5): 1192-1198.
CHEN Liang, TANG Xin-cun, ZHANG Yang, QU Yi, WANG Zhi-min. Separation and recovery of cobalt, nickel and manganese from waste lithium ion batteries[J]. Journal of Chinese Nonferrous Metals, 2011, 21(5): 1192-1198.
[15] YANG Y, XU S M, HE Y H. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes[J]. Waste Management, 2017, 64: 219-227.
[16] LI L, FAN E S, GUAN Y B, ZHANG X, XUE Q, WEI L, WU F, CHEN R J. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5224-5233.
[17] GUO Y, LI F, ZHU H C, LI G M, HUANG J W, HE W Z. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl)[J]. Waste Management, 2016, 51: 227-233.
[18] HU J T, ZHANG J L, LI H X, CHEN Y Q, WANG C Y. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries[J]. Journal of Power Sources, 2017, 351: 192-199.
[19] HE L P, SUN S Y, MU Y Y, SONG X F, YU J G. Recovery of lithium, nickel, cobalt, and manganese from spent lithium-ion batteries using l-tartaric acid as a leachant[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(1): 714-721.
[20] CHEN X P, MA H R, LUO C B, ZHOU T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid[J]. Journal of Hazardous Materials, 2017, 326: 77-86.
[21] XIN Y Y, GUO X M, CHEN S, WANG J, WU F, XIN B P. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery[J]. Journal of Cleaner Production, 2016, 116: 249-258.
[22] HONG H S, KIM D W, CHOI H L, RYU S S. Solvent extraction of Co, Niand Mn from NCM sulfate leaching solution of Li(NCM)O2 secondary battery scraps[J]. Archives of Metallurgy and Materials, 2017, 62(2): 1011-1014.
[23] NAYL A A, HAMED M M, RIZK S E. Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 55: 119-215.
[24] 潘晓勇, 彭 玲, 陈伟华, 韦泽平, 卢 潇. 废旧锂离子电池中钴和锂的回收及综合利用[J]. 中国有色金属学报, 2013, 23(7): 2047-2054.
PAN Xiao-yong, PENG Ling, CHEN Wei-hua, WEI Ze-ping, LU Xiao. Recovery and comprehensive utilization of cobalt and lithium in waste lithium ion batteries[J]. Journal of Chinese Nonferrous Metals, 2013, 23(7): 2047-2054.
[25] CHEN X P, FAN B L, XU L P, ZHOU T, KONG J R. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries[J]. Journal of Cleaner Production, 2016, 112: 3562-3570.
[26] MESHRAM P, PANDEY B D, MANKHAND T R. Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching[J]. Chemical Engineering Journal, 2015, 281: 418-427.
[27] 诸爱士, 徐 亮, 沈芬芳, 成 忠. 钴与镍的分离技术研究综述[J]. 浙江科技学院学报, 2007, 19(3): 169-174.
ZHU Ai-shi, Xu Liang, SHEN Fen-fang, CHENG Zhong. Review of separation technology of cobalt and nickel[J]. Journal of Zhejiang University of Science and Technology, 2007, 19(3): 169-174.
Separation and recycling of cobalt, nickel, manganese and lithium from acidic leaching solution of old ternary battery positive active material
JIANG Ling1, ZHAN Lu1, 2, ZHANG Qiu-zhuo1, 3
(1. School of Ecological and Environmental Sciences, East China Normal University, Shanghai 200241, China;
2. School of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
3. Institute of Eco-Chongming(IEC), Shanghai 200062, China)
Abstract: The metal ions of most solid powders in the cathode active materials of waste ternary batteries can be transferred to the acid solution by sulfuric acid leaching agent. In this work, the separation and recovery of cobalt, nickel, manganese and lithium in sulfuric acid leaching solution were studied. The proposed precipitation process was precipitated by ammonium oxalate precipitation method-precipitation of cobalt by ammonium bicarbonate precipitation method-precipitation of nickel by sodium hydroxide-sodium hydroxide coprecipitation method-precipitation of lithium by sodium carbonate. The separation conditions were optimized by changing the pH value, temperature, stirring time and amount of precipitant. The experimental results show that under the optimized process conditions, the metal ions in the solution are precipitated in the form of cobalt oxalate, manganese carbonate, basic nickel carbonate and lithium carbonate. The recovery rates of cobalt, manganese, nickel and lithium are 99.17%, 97.88%, 93.47% and 85.21%, respectively. The mass fractions of cobalt oxalate, manganese carbonate, basic nickel carbonate and lithium carbonate are 98.77%, 98.89%, 98.46% and 96.52%, respectively.
Key words: ternary lithium battery; sulfuric acid leaching solution; cobalt nickel manganese lithium; precipitation separation
Received date: 2019-10-21; Accepted date: 2020-08-10
Corresponding author: ZHAN Lu; Tel: +86-21-54747495; E-mail: luzhan@sjtu.edu.cn
(编辑 王 超)
收稿日期:2019-10-21;修订日期:2020-08-10
通信作者:詹 路,副教授,博士;电话:021-54747495;E-mail:luzhan@sjtu.edu.cn