J. Cent. South Univ. Technol. (2008) 15(s1): 102-106
DOI: 10.1007/s11771-008-324-0
Rheological properties of novel thermo-responsive polycarbonates
aqueous solutions
WANG Yue-xia(王月霞)1, TAN Ye-bang(谭业邦)1, 2, HUANG Xiao-ling(黄晓玲)1
(1. School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China;
2. Key Laboratory of Colloid and Interface Chemistry, Ministry of Education, Shandong University,
Jinan 250100, China)
Abstract: Thermo-responsive multiblock polycarbonates were facilely synthesized by covalently binding poly(ethylene glycol) (PEG) and poly(propylene glycol) (PPG) blocks, using triphosgene as coupling agent and pyridine as catalyst.The aqueous solutions of thermo-responsive polycarbonates were investigated by rheological measurements. Steady-state shear measurements reveal that the polycarbonate solutions exhibit shear-thinning behavior and the hydrophilic content has a pronounced effect on the flow behavior of the polycarbonates aqueous solutions. The shear viscosity decreases with increasing poly(ethylene oxide) (PEO) composition. The increase of viscosity with increasing concentration is probably attributed to the formation of stronger network owing to interchain entanglement of PEO block at higher concentration. When the flow curves are fitted to the power law model, flow index is obtained to be less than 1, as exhibiting typical pesudoplastic fluid. The viscoelastic properties of the system also show close dependence on the composition of polycarbonates. Temperature sweep confirms that the multiblock polycarbonates exhibit thermo-responsive properties. For 7% aqueous solution of polycarbonate with composition ratio of EO to PO of 1/1, the sol-gel transition occurs at 37 ℃, which makes the system suitable as an injectable drug delivery system.
Key words: polycarbonates; thermo-responsive; rheological measurements; viscosity; gel
1 Introduction
Thermo-responsive amphiphilic copolymers have been studied extensively as potential biomaterials in the past decade[1-4]. Particularly, polymers with capability of sol-to-gel transition by temperature change constitute an active area of research due to their wide applications in drug delivery and tissue engineering. In previous publications, numerous studies have been performed to investigate the solution behavior of poly(ethylene oxide)-poly(propylene oxide)-poly (ethylene oxide) (PEO-PPO-PEO) triblock copolymers (commercially available from BASF Crop., under the name Pluronics)[5-7]. Most studies have focused on the sol-to-gel transition of Pluronic F127 (EO99PO67- EO99)[8-10]. Generally, both PEO and PPO are water soluble at low temperature thus the F127 triblock copolymer chains are well miscible in aqueous solution. When the temperature was elevated, the PPO block was no longer hydrated and became hydrophobic. As a result, micelles composed of a PPO core and a PEO corona began to form as the concentration was above critical micellization concentration (CMC)[11]. As the concentration and/or temperature increased further, the
micelles came into contact with one another and then these contacts ultimately caused entanglement among the hydrophilic PEO blocks. Finally, the formation of a gel structure occurred[6-10]. Regrettably, the gels obtained from solutions of these PEO-PPO-PEO triblocks were found to dissolve in vivo in a short time upon dilute, resulting in the systems exhibited limited stability and short resident times[12]. Furthermore, the triblock copolymers are toxic and nondegradable. These drawbacks limit most of their applications in many fields, such as tissue engineering and implantation where high elastic modulus and biodegradable are needed.
In order to improve the mechanical properties of the gel, while still keeping the thermo-responsivity, novel multiblock polycarbonates were synthesized by covalently binding PEG and PPG, using triphosgene as coupling agent and pyridine as catalyst. In this way, a new kind of thermo-responsive gel was made and by adjusting the EO/PO composition, gel phase was formed at about 37 ℃. Moreover, the existence of carbonate linkage in the polycarbonates makes it degradable since the carbonate bindings are hydrolyzed under physiological conditions. These allow the system promising for use in biomedical applications.
2 Experimental
2.1 Synthesis of multiblock polycarbonate
The novel multiblock thermo-responsive polycarbonates possessing the following structure were synthesized. The synthesis of PEG7000/PPG2000 multiblock polycarbonates is shown in Eqn.(1). Three polycarbonates with initial feed molar ratios of PEG to PPG of 0.8/1, 0.9/1 and 1/1 were obtained and they were signed as PC1, PC2 and PC3, respectively.
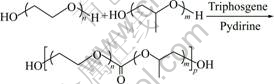 (1)
(1)
2.2 Preparation of polycarbonate solution
Homogeneous solutions were prepared by slowly adding predetermined amount of polycarbonates to cold distilled water followed by stirring. Then the sample solutions were kept at 4 ℃ for 24 h to equilibrate before the rheological measurements.
2.3 Rheological measurements
Rheological properties were measured using a HAAKE RS75 Rheometer equipped with a cone-plate geometry C20/1*Ti. Steady-state shear measurements were carried out over a period of 180 s at 25 ℃. Stress sweeps were conducted at a fixed frequency of 1.0 Hz to obtain the linear viscoelastic range. After a proper stress was selected, the frequency sweeps were performed with a frequency range of 0.1-25 Hz and temperature sweeps with temperature ranging from 15 ℃ to 70 ℃.
3 Results and discussion
3.1 Effect of composition on flow behavior of polycarbonate solution
The flow curves of 15% (mass fraction) aqueous solution for polycarbonates with various EO/PO composition measured at 25 ℃ are shown in Fig.1. All the samples exhibit shear-shinning behavior, which is the typical behavior of pseudoplastic fluid. The shear stress τ increases gradually with shear rate ε and the increase rate differentiates for different polycarbonates. Concomitantly, it is clear that both shear viscosity and shear stress values decrease as the composition of PEO blocks increase. This suggests that the hydrophilic content has a pronounced effect on the flow behavior of the polycarbonates aqueous solution. The shear viscosity decreases with increasing EO composition. In order to further verify this point of view, the flow behavior of PC1 and PC2 at different concentrations was investigated.
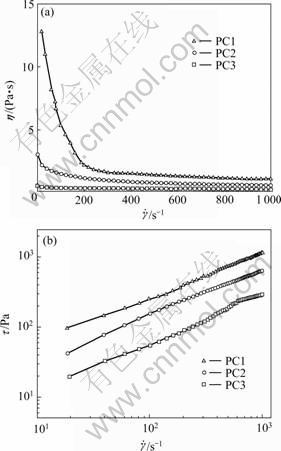
Fig.1 Flow curves of 15% aqueous solutions for polycarbonates with different compositions at 25 ℃
3.2 Effect of concentration on flow behavior of polycarbonate solution
The curves of flow behavior of polycarbonates at different concentrations are shown in Fig.2. The change of shear stress over the applied shear rate range for both of the polycarbonates at each concentration appears almost the same, which means that all the solutions at the concentration range measured behaves like pesudoplastic fluid. For each polycarbonate, with increasing concentration, the shear stress increases. This is probably attributed to the formation of stronger network owing to interchain entanglement of PEO block at higher concentration. The network structure is broken down when the shear rate increases, so the shear thinning behavior is observed as shown in Fig.1(a).
Since the polycarbonate solutions show non-Newtonian behavior, the steady state shear flow curves can be simulated by the following power law model.
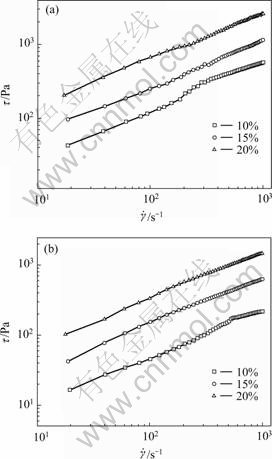
Fig.2 Flow curves of aqueous solution for polycarbonates PC1(a) and PC2(b) at different concentrations at 25 ℃
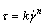 (2)
(2)
where τ is shear stress; 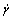 is shear rate; k is viscosity coefficiency and n is flow index. If n = 1, the viscosity of system is independent of shear rate (equal to k) and the system is regarded as Newtonian fluid. In the case of n>1, the system behaves shear-thickening, and in the case of n<1 just as in the present case, the system exhibits shear-thinning behavior. Then the rheological parameters of the system are calculated as shown in Fig.3. All the system has a flow index of less than 1, implying that the rheological behavior of the system deviates from Newtonian fluid. With increasing concentration of the polycarbonates, the flow index decreases. However, the viscosity coefficient increases. A high viscosity coefficient implies that the samples at high concentration display high shear viscosity. Moreover, it is observed that the values of flow index for PC1 are smaller than those for PC2 at other similar condition, which suggests that PC1 system shows stronger shear-thinning behavior than PC2. Whereas the values of viscosity coefficient for PC1 are larger than those of PC2, implying that higher viscosity are obtained from PC1 system.
is shear rate; k is viscosity coefficiency and n is flow index. If n = 1, the viscosity of system is independent of shear rate (equal to k) and the system is regarded as Newtonian fluid. In the case of n>1, the system behaves shear-thickening, and in the case of n<1 just as in the present case, the system exhibits shear-thinning behavior. Then the rheological parameters of the system are calculated as shown in Fig.3. All the system has a flow index of less than 1, implying that the rheological behavior of the system deviates from Newtonian fluid. With increasing concentration of the polycarbonates, the flow index decreases. However, the viscosity coefficient increases. A high viscosity coefficient implies that the samples at high concentration display high shear viscosity. Moreover, it is observed that the values of flow index for PC1 are smaller than those for PC2 at other similar condition, which suggests that PC1 system shows stronger shear-thinning behavior than PC2. Whereas the values of viscosity coefficient for PC1 are larger than those of PC2, implying that higher viscosity are obtained from PC1 system.

Fig.3 Effect of concentrations on rheological constant of polycarbonate systems: (a) PC1; (b) PC2
3.3 Effect of composition on viscoelastic properties of polycarbonate solution
In order to determine the linear viscoelastic region in the oscillatory test, a fixed frequency of 1.0 Hz was used while a stress sweep was performed. A typical result of complex modulus G* as a function of the stress for 20% polycarbonates system at 25 ℃ is shown in Fig.4.

Fig.4 Stress sweeping curves of 20% polycarbonate aqueous solutions at 1.0 Hz and 25 ℃
It can be seen that for PC1 and PC2 the values of G* remain virtually constant with increasing stress τ until the critical stress value τc is reached, after which G* begins to decrease with further increase in the applied stress. The region where G* is independent on stress is regarded as linear viscoelastic region. However, the values of G* for PC3 show noticeable fluctuation which reveals that the viscous component maintains dominating in 20% aqueous solution of PC3.
Fig.5 illustrates the storage modulus G′ and loss modulus G" for 20% PC2 aqueous solution as a function of frequency. It reveals that both G" and G′ increase with increasing frequency in the frequency range measured. At low frequencies, G′ is less than G′′, indicating that the system display a liquid-like behavior. As the frequency is increased to about 20 Hz, G′ becomes larger than G′′, the system behaves as elastic material. Thus it can be concluded that the viscoelastic behavior of the system is more predominant at high frequency range, which may be due to the formation of networks containing entangled or interconnected chains.
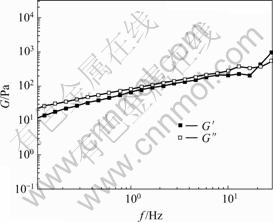
Fig.5 Frequency sweeping curves of 20% PC2 aqueous solutions at 25 ℃
3.4 Effect of temperature on viscoelastic properties of polycarbonate solution
The temperature dependence of G′ and G" for 7% PC1 solution is shown in Fig.6. Both G′ and G" exhibit a fairly increase as initial heating up to about 25 ℃ and then they increase remarkably, during this process G′ is less than G". However, G′ increases more sharply than G" with further heating. When the temperature reaches about 37 ℃ as shown in the insert curves of Fig.6, a crossover of G′ and G" appears, indicating that the sample undergoes a sol-gel transition, forming an elastic network, after which the enhancement of G′ and G" can be observed with elevated temperature in the temperature range investigated. Interestingly, the sol-to-gel transition for 7% aqueous solution is detected at around body temperature, which makes this system suitable as injectable drug delivery systems.

Fig.6 Temperature sweeping curves of 7% PC1 aqueous solutions at 1.0 Hz
4 Conclusions
1) Thermo-responsive PEO/PPO multiblock polycarbonates were synthesized through one-step condensation copolymerization using triphosgene as coupling agent and pyridine as catalyst. The resulting polycarbonates were biodegradable due to the existence of carbonate linkage.
2) Steady-state shear measurements show that the polycarbonate solution exhibits shear-thinning behavior. Increasing content of PEO blocks decreases the shear viscosity, whereas the increase of concentration increases the shear viscosity. The flow index of the system is found to be less than 1 as shown by typical pseudoplastic fluid.
3) The viscoelastic properties can also be affected by polycarbonates composition. For polycarbonate with EO/PO composition ratio of 1/1, by adjusting the concentration of polycarbonate aqueous solution to 7%, gel phase appears with increasing the temperature to 37 ℃, implying that the system is promising for use in biomedical applications.
References
[1] HE Han-wei, LIU Hong-jian, ZHOU Ke-chao, WANG Wei, RONG Peng-fei. Characteristics of magnetic Fe3O4 nanoparticles encapsulated with human serum albumin [J]. J Cent South Univ Technol, 2006, 13(1): 6-11.
[2] ZHAO Guang-qiang, CHEN S B. Phase behavior and shear thickening HM-HEC solutions with addition of nonionic surfactant C12E5 [J]. J Cent South Univ Technol, 2007, 14(1): 202-205.
[3] CHEN G H, HOFFMAN A S. Graft-copolymers that exhibit temperature-induced phase-transition over a wide-range of pH [J]. Nature, 1995, 373(6509): 49-52.
[4] THOMAS J L, YOU H, TIRRELL D A. Tuning the response of a pH-sensitive membrane switch [J]. J Am Chem Soc, 1995, 117(10): 2949-2950.
[5] PARK M J, CHAR K. Two gel states of a PEO-PPO-PEO triblock copolymer formed by different mechanisms [J]. Macromol Rapid Commum, 2002, 23(12): 688-692.
[6] NYSTROM B, WALDERHAUG H. Dynamic viscoelasic of an aqueous system of a poly(ethylene oxide)-poly(propylene oxide)- poly(ethylene oxide) triblock copolymer during gelation [J]. J Phys Chem, 1996, 100(13): 5433-5439.
[7] JORGENSEN E B, HVIDT S, BROWN W, SCHILLEN K. Effect of salts on the micellization and gelation of a triblock copolymer studied by rheology and light scattering [J]. Macromolecules, 1997, 30(8): 2355-2364.
[8] MORTENSEN K, BROWN W, NORDEN B. Inverse melting transition and evidence of three-dimensional cubatic structure in a block copolymer micellar system [J]. Phys Rev Lett, 1992, 68(15): 2340-2343.
[9] MORTENSEN K, PEDERSEN J S. Structural study on the micelle formation of poly(ethylene oxide)-poly(propylene oxide)-poly (ethylene oxide) triblock copolymer in aqueous solution [J]. Macromolecules, 1993, 26(4): 805-812.
[10] WANAKA G, HOFFMANN H, ULBRICHT W. The aggregation behavior of poly-(oxyethylene)-poly-(oxyproprylene)-poly (oxyethylene)-block-copolymers in aqueous solution [J]. Colloid Polym Sci, 1990, 268(2): 101-117.
[11] ALEXANDRIDIS P, HOLZWARTH J F, HATTON T A. Micellization of poly(ethylene oxide)-poly(propylene oxide)- poly(ethylene oxide) triblock copolymers in aqueous solutions: thermodynamics of copolymer association [J]. Macromolecules, 1994, 27(9): 2414-2425.
[12] JEONG B, BAE Y H, KIM S W. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof [J]. J Biomed Mater Res, 2000, 50(2): 171-177.
(Edited by LONG Huai-zhong)
Foundation item: Projects(2006GG2203007) supported by the Scientific Research Project of Shandong Province, China
Received date: 2008-06-25; Accepted date: 2008-08-05
Corresponding author: TAN Ye-bang, Professor; Tel: +86-531-88363502; E-mail: ybtan@sdu.edu.cn