Article ID: 1003-6326(2005)05-1145-05
Behavior of calcium silicate hydrate in aluminate solution
LI Xiao-bin(李小斌), ZHAO Zhuo(赵 卓), LIU Gui-hua(刘桂华),
ZHOU Qiu-sheng(周秋生), PENG Zhi-hong(彭志宏)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Using calcium hydroxide and sodium silicate as starting materials, two kinds of calcium silicate hydrates, CaO·SiO2·H2O and 2CaO·SiO2·1.17H2O, were hydro-thermally synthesized at 120℃.The reaction rule of calcium silicate hydrate in aluminate solution was investigated. The result shows that CaO·SiO2·H2O is more stable than 2CaO·SiO2·1.17H2O in aluminate solution and its stability increases with the increase of reaction temperature but decreases with the increase of caustic concentration. The reaction between calcium silicate hydrate and aluminate solution is mainly through two routes. In the first case, Al replaces partial Si in calcium silicate hydrate, meanwhile 3CaO·Al2O3·xSiO2·(6-2x) H2O (hydro-garnet) is formed and some SiO2 enters the solution. In the second case, calcium silicate hydrate can react directly with aluminate solution, forming hydro-garnet and Na2O·Al2O3·2SiO2·nH2O (DSP). The desilication reaction of aluminate solution containing silicate could contribute partially to forming DSP.
Key words: calcium silicate hydrate; aluminate; caustic; reaction behavior CLC number: TF802
Document code: A
1 INTRODUCTION
Calcium silicate hydrate (mCaO·nSiO2·xH2O, C-S-H) is an important hydrate product of Portland cement. Because of its key rules in setting and hardening performance of cement[1], it has attracted a lot of interest of many researchers[2-4]. Calcium silicate hydrate is also a very important substance in alumina production, but its reaction behavior with aluminate solution has not been paid adequate emphases upon.
The key project in alumina production is the separation of aluminum and silicon. Calcium silicate hydrate is an important compound containing silicon in the process of alumina production. In order to separate aluminum and silicon, the silicate compounds without or with less alumina, such as C-S-H, are formed in high-pressure hydro-chemical process under very rigorous condition of high temperature and high caustic concentration[5, 6]. A method to obtain C-S-H from sodium alumino-silicate hydrate (DSP) under mild condition was put forward[7, 8]. Moreover, in sintering process of alumina production, the secondary loss of alumina and soda is actually caused by the reactions between calcium silicate hydrate and aluminate solution in the operation of leaching sinter[9]. Therefore it is of great importance for alumina production to know the reaction law between C-S-H and aluminate solution.
The reactions between C-S-H and aluminate solution are generally considered as follows[9]:
mCaO·nSiO2·xH2O+mNa2CO3=
nNa2SiO3+mCaCO3+2(m-n)NaOH+
(n-m)H2O(1)
mCaO·nSiO2·xH2O+2nNaOH=
mCa(OH)2+nNa2SiO3+
(x+n-m)H2O(2)
3Ca(OH)2+2NaAl(OH)4+xNa2SiO3=
3CaO·Al2O3·xSiO2·(6-2x)H2O+
2(1+x)NaOH+xH2O(3)
2Na2SiO3+2NaAl(OH)4=
Na2O·Al2O3·2SiO2·xH2O+
4NaOH+(2-x)H2O(4)
Conclusions can be drawn from the above reactions, i.e., the decomposition reactions of C-S-H with Na2CO3 and NaOH in aluminate solution (reactions (1) and (2)) are the basis of forming hydro-garnet and DSP (reactions (3) and (4)). The concentration of SiO2 in the solution from leaching sinter can reach 4-6g/L, which is generally considered the decomposition of C-S-H by Na2CO3 and especially by NaOH[9, 10]. However, Liu et al[11] experimentally studied the reaction behavior of C-S-H with Na2CO3 and NaOH and the results showed that the SiO2 concentration in the obtained solution is only 0.1-0.5g/L in both NaOH and Na2CO3 system with concentration of 100g/L Na2O. The SiO2 concentration is much less than that in sinter leaching, indicating the limited reaction degree of C-S-H with Na2CO3 and NaOH. Therefore the reaction mechanism between C-S-H and aluminate solution is not clear and further investigation is necessary.
2 METHODOLOGY
Firstly, the possibility of the reaction between C-S-H and aluminate solution is analyzed thermodynamically, and then experiments are carried out to validate the thermodynamic conclusion. Calcium hydroxide and sodium silicate are used as starting materials to synthesize two kinds of calcium silicate hydrate at 120℃, respectively being CaO·SiO2·H2O (signed as CSH1) and 2CaO·SiO2·1.17H2O (signed as CSH2). The content of dissociated CaO in both CaO·SiO2·H2O and 2CaO·SiO2·1.17H2O are measured as respectively 0.3% and 0.7% by glycol-ethanol means[12]. The loss of alumina in solution, caused by the formation of 3CaO·Al2O3·xSiO2·(6-2x)H2O due to the reactions of those CaO with aluminate, is not significant (respectively 0.018g/L and 0.4g/L) and can be ignored. The samples are then treated in aluminate solution with the caustic ratio αk of 1.5(Na2O/Al2O3, molar ratio)at L/S of 10 (mass ratio of liquid to solid), unless especially noted. The specimens are taken out and filtered at designed time to measure the concentration of SiO2 and Al2O3 in filtrate and mineral phase in residue.
3 RESULTS AND DISCUSSION
3.1 Thermodynamic analysis
The high SiO2 concentration in the solution after the reaction of C-S-H and aluminate solution means that the substitution reaction of Al for Si, only in bridging sites of the dreierkette[13], may happen as follows:
3[mCaO·nSiO2·xH2O]+2mAl(OH)-4+
(m-3x)H2O=m[3CaO·Al2O3·
SiO2·4H2O]+(3n-m)H2SiO2-4+
(4m-6n)OH-(5)
The XRD patterns reveal that DSP emerges at the beginning of the reaction of C-S-H and aluminate solution, which means that the formation of DSP may be not only caused by the reaction between H2SiO2-4 from reaction (5) and Al(OH)-4 in solution, but also by the following reaction regarding to Ref.[9]:
3[mCaO·nSiO2·xH2O]+(2m+3n-m)·
Al(OH)-4+(3n-m)Na+=m[3CaO·
Al2O3·SiO2·4H2O]+(3n-m)/2·
[Na2O·Al2O3·2SiO2·2H2O]+
2mOH-+(3n+3x-2m)H2O(6)
The calculation results of dependence of Gibbs free energy change on temperature for reactions (5) and (6) have been carried out with the Alumina Thermodynamic Database[14]. Fig.1 shows that it is possible for both CaO·SiO2·H2O and 2CaO·SiO2·1.17H2O to react with Al(OH)-4 according to reaction (5), but impossible for 6CaO·6SiO2·H2O. Meanwhile, all the C-S-H are able to react with aluminate solution on the basis of reaction (6). Thermodynamically speaking, the stability sequence of the calcium silicate hydrates mentioned above is 6CaO·6SiO2·H2O>CaO·SiO2·H2O>2CaO·SiO2·1.17H2O.

]Fig.1 Variation of ΔG versus temperature
3.2 Relationship between types of C-S-H and its stability
Fig.2 demonstrates the loss of alumina due to SiO2(mAl2O3) and the dissolution of SiO2(ηSiO2) versus time in the reaction of the two types of C-S-H with aluminate solution. Fig.2 indicates that the stability of the two types of C-S-H are quit different in aluminate solution. The mAl2O3 of CaO·SiO2·H2O is obviously less than that of 2CaO·SiO2·1.17H2O and the ηSiO2 of the later is more than that of the former. Thus it can be concluded that CaO·SiO2·H2O is more stable than 2CaO·SiO2·1.17H2O, being consistent with the thermodynamic calculation. This can be explained as that there are silicate anion chains in all C-S-H and these chains become progressively longer as the Ca/Si ratio decreases, which causes the enhancement of the aggregation degree of Si and thus the increment of stability of C-S-H[15].
3.3 Influence of temperature on stability of C-S-H
Fig.3 indicates the effect of temperature on ηSiO2 in the case of the same aluminate solution and tells that the stability of C-S-H depends on temperature. The change of Gibbs free energy for the reactions increases with the elevation of temperature, resulting in the decrease of ηSiO2 and thus C-S-H becomes more stable in aluminate solution at higher temperature. 3.4 Influence of composition of aluminate solution on stability of C-S-H
3.4.1 Caustic(Na2Ok) concentration
Fig.4 depicts the results of the reaction between CaO·SiO2·H2O and aluminate solution with different Na2Ok concentrations. The concentration of SiO2 entering the solution remarkably increases with the augment of Na2Ok concentration and has a maximum value. The high Na2Ok concentration represents a high Al(OH)-4 concentration at a certain αk which is beneficial to SiO2 in C-S-H entering the solution according to reaction (5). Subsequently, after the SiO2 exceeds its equilibrium, the concentration of SiO2 in the solution will drop due to the formation of DSP produced by the reaction of H2SiO2-4 and the components of solution. This process, together with reactions (5) and (6), will contribute to the loss of alumina, mAl2O3. On the contrary, there is a low concentration of Al(OH)-4 in the system with low Na2Ok concentration (50g/L) and thus a limited reaction degree, which results in a less and invariable SiO2 concentration in the solution at any time.

Fig.2 Loss of alumina (a) and dissolution of SiO2(b) versus time
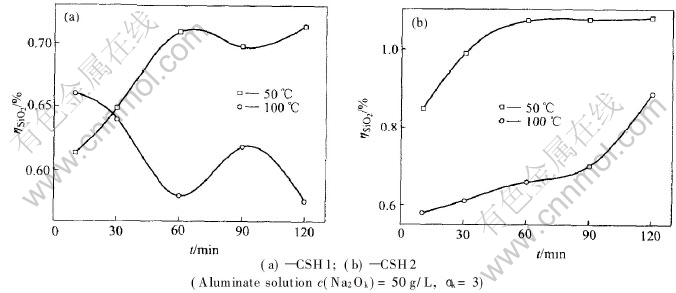
Fig.3 Dissolution of SiO2 versus time
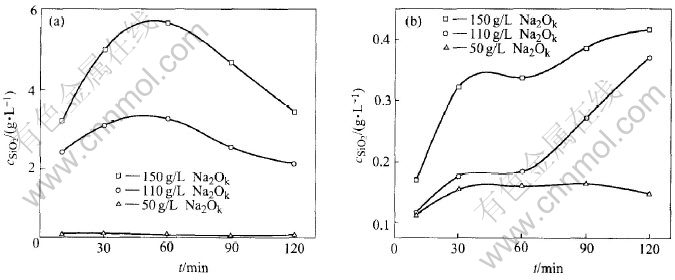
Fig.4 SiO2 concentration in solution(a) and loss of alumina(b) versus time
3.4.2 Concentration of Na2CO3
Fig.5 shows the result of the reaction of two kinds of C-S-H and aluminate solution in different Na2CO3 concentrations. We can conclude from the figure that the dependence of ηSiO2 on Na2CO3 concentration for C-S-H is not significant, indicating a less effect of Na2CO3 concentration on the stability of C-S-H.

Fig.5 Dissolution of SiO2 versus time
3.4.3 Caustic ratio(αk)
From Fig.6 we can know that the higher the αk of aluminate solution is, the more the SiO2 in C-S-H dissolves. Obviously, the free NaOH concentration in the aluminate solution with αk=3.0 is higher than that in the aluminate solution with αk=1.5 in a certain caustic concentration. The concentration of SiO2 entering the solution in the former solution should be more than that in the later, which is not identical with the fact, if the decomposition of C-S-H is mainly through reaction (2). Combining what mentioned above and Fig.5, the conclusion that reactions (1) and (2) contribute less than reaction (5) to the higher SiO2 concentration in the sinter leaching operation in the sintering process, can be drawn.
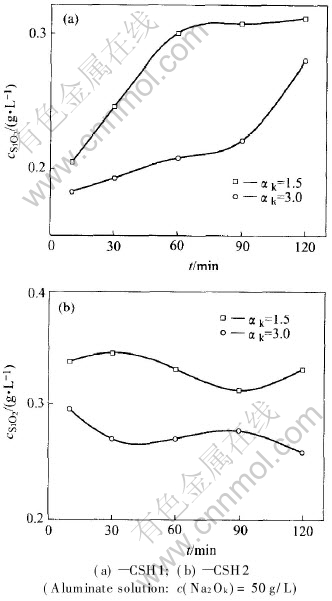
Fig.6 SiO2 concentration in solution versus time
4 CONCLUSIONS
1) Thermodynamically, CaO·SiO2·H2O is more stable than 2CaO·SiO2·1.17H2O in aluminate solution and the stability of C-S-H increases with the elevation of temperature, which has been verified by experiments.
2) The concentrations of Na2CO3 and NaOH have less influence on the stability of calcium silicate hydrates; meanwhile the Al(OH)-4 concentration in aluminate solution affects those remarkably through the formation of hydro-garnet and sodium alumino-silicate hydrate.
3) Al(OH)-4 contributes more significantly than NaOH and Na2CO3 on the high SiO2 concentration in the solution after the reaction of calcium silicate hydrates with aluminate solution.
REFERENCES
[1]Garrault-Gauffinet S, Nonat A. Experimental investigation of calcium silicate hydrate(C-S-H) nucleation [J]. Journal of Crystal Growth, 1999, 200: 565-574.
[2]Viehland D, LI Jie-fang, YUAN Li-jian. Mesostructure of calcium silicate hydrate(C-S-H) gels in Portland cement paste: short-range ordering, nanocrystallinity, and local compositional order [J]. J Am Ceram Soc, 1996, 79(7):1731-1744.
[3]CONG Xian-dong, James K R. 29Si MAS NMR study of the structure of calcium silicate hydrate [J]. Advn Cem Bas Mat, 1996, 3: 144-156.
[4]Moropoulou A, Cakmak A, Labropoulos K C. Accelerated microstructural evolution of a calcium-silicate-hydrate(C-S-H) phase in pozzolanic pastes using fine siliceous sources: comparison with historic pozzolanic mortars [J]. Cement and Concrete Research, 2004, 34: 1-6.
[5]Sazhin V S. Advance of Hydrothermal Chemical Process for Alumino-silicate Mineral and Bauxite with much Silicon [M]. MOSKVA, Metallurgical, 1989. 63-100.(in Russian)
[6]SHAO Zhi-bo. The development way of Chinese alumina industry[J]. World Nonferrous Metals, 1999(3): 8-12.(in Chinese)
[7]ZHANG Ya-li, LIU Xiang-min, PENG Zhi-hong. Study on the wet treatment hydrate alunino silicate—alkali recovery [J]. Mining and Metallurgical Engineering, 2003, 6(23): 56-58.(in Chinese)
[8]LIU Gui-hua, ZHANG Ya-li, PENG Zhi-hong. Alumina recovery from sodium hydrate alumino-silicate [J]. The Chinese Journal of Nonferrous Metals, 2004, 3(14): 499-503.(in Chinese)
[9]YANG Zhong-yu. The Technology of Alumina Production [M]. Beijing: Metallurgical Industry Press, 1982: 254-265.(in Chinese)
[10]YUAN Yi, XIANG Yang, HUANG Fang. An elementary analysis on the secondary of leaching sintering mixture of aluminum[J]. Light Metals, 1999, 12: 18-21.(in Chinese)
[11]LIU Gui-hua, LI Xiao-bin, PENG Zhi-hong. Exploration of reaction mechanism of calcium silicate hydrate in basic solution [J]. Journal of the Chinese Ceramic Society, 2004, 32(6): 777-780.(in Chinese)
[12]GONG Jin-sheng, DENG Zhao-ying. A method to measure the dissociated CaO in the sinter quickly: glycol-ethanol means [J]. Cement, 1987(8): 31-32.(in Chinese)
[13]Richardson G, Brough R, Brydson R. Location of aluminum in substituted calcium silicate hydrate(C-S-H) gels as determined by 29Si and 27Al NMR and EELS [J]. J Am Ceram Soc, 1993, 76(9): 2285 -2288.
[14]LI Yong-fang. Thermodynamics Database for Alumina Production [D]. Changsha: Central South University, 2001.(in Chinese)
[15]Yoshihiko O. 29Si NMR spectroscopy of silicate anions in hydro-thermally formed C-S-H [J]. J Am Ceram Soc, 1994, 77(3): 765-768.
Foundation item: Project(50274076) supported by the National Natural Science Foundation of China
Received date: 2005-01-27; Accepted date:2005-07-07
Correspondence: LI Xiao-bin, Professor, PhD; Tel: +86-731-8830453; E-mail: X.B.Li@mail.csu.edu.cn
(Edited by YANG Bing)